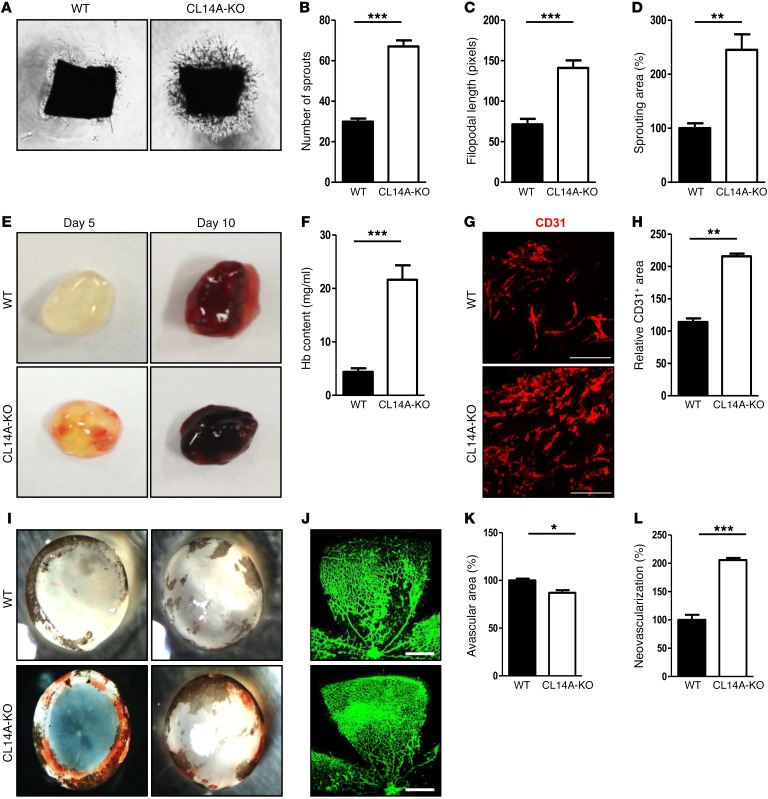
Figure 2. Deletion of Clec14a results in increased development of poorly functional (hemorrhage-prone) vessels.
(A) Murine aortic ring assay for P7 WT and CLEC14A-KO mice (VEGF-A; 50 ng). n = 3 per group. Magnification ×10. (B–D) Quantification of the number of sprouts, filopodial length, and relative sprouting area (percentage of control). (E) Matrigel plugs implanted into 7-week-old WT and CLEC14A-KO mice for 5 or 10 days. n = 6 per group. (F) Quantification of Hb (mg/ml) extracted from Matrigel plugs from WT and CLEC14A-KO mice. n = 6 per group. (G) CD31 immunostaining of Matrigel plugs implanted into WT and CLEC14A-KO mice for 10 days. Scale bars: 100 μm. (H) Quantification of the relative CD31-positive area (percentage of control). (I) Hemorrhage in P17 WT and CLEC14A-KO mouse retinae after hyperoxia (OIR model). n = 3 per group. (J) Whole-mount isolectin B (green) staining of P17 retinae from WT and CLEC14A-KO mice. Scale bars: 500 μm. (K and L) Relative avascular and neovascularization areas in P17 retinae from WT and CLEC14A-KO mice (percentage of control). All experiments were repeated on at least 3 different sets of WT and KO littermates. *P < 0.05, **P < 0.005, and ***P < 0.0001, by paired, 2-tailed Student’s t test. Error bars represent the mean ± SD.