Abstract
Background
The GABAergic neuroactive steroid (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP, allopregnanolone) enhances GABAergic activity and produces subjective effects similar to ethanol. The effect of chronic alcohol exposure on 3α,5α-THP concentrations has been studied in mouse, rat, and monkey limbic brain areas. Chronic ethanol exposure produced divergent brain region and cell specific changes in 3α,5α-THP concentrations in animal studies. However, 3α,5α-THP levels in similar human brain regions have never been examined in individuals diagnosed with alcohol use disorder (AUD). Therefore, we used immunohistochemistry to examine 3α,5α-THP levels in the ventral tegmental area (VTA), substantia nigra pars medialis (SNM), and amygdala of human postmortem brains of patients diagnosed with AUD compared to social drinkers. The effects of sex and liver disease on 3α,5α-THP concentrations were examined in the aforementioned brain regions.
Methods
Human postmortem brains of AUD patients and age-matched controls were obtained from the New South Wales Brain Tissue Resource Center. Immunohistochemistry was performed using anti-3α,5α-THP antibody on formalin fixed and paraffin embedded brain sections to detect cellular 3α,5α-THP levels. Immunoreactivity was analyzed by pixel density/mm2 for the comparison between AUD patients and controls.
Results
3α,5α-THP immunoreactivity was increased by 23.2±9% in the VTA of AUD patients compared to age matched controls (p= 0.014). Moreover, a 29.6±10% increase in 3α,5α-THP immunoreactivity was observed in the SNM of male AUD patients compared to male controls (p<0.01), but not in female subjects. 3α,5α-THP immunoreactivity in the VTA and SNM regions did not differ between non-cirrhotic and cirrhotic AUD patients. A sex difference in 3α,5α-THP immunoreactivity (female 51±18% greater than male) was observed among control subjects in the SNM, but no other brain region. 3α,5α-THP immunoreactivity in the basolateral and lateral amygdala were negatively correlated with the length of the tissue fixation time as well as the age of the subjects, precluding assessment of the effect of AUD.
Conclusions
Cellular 3α,5α-THP levels in VTA are increased in human AUD patients, an effect that is likely independent of sex and liver disease. The differences between animal models and human studies should be factored into the interpretation of the physiological significance of elevated 3α,5α-THP levels in humans.
Keywords: Alcohol; 3α,5α-THP; allopregnanolone; neuroactive steroid; postmortem human
Introduction
Alcohol Use Disorder (AUD) is a chronic relapsing disorder that is one of the leading causes of lost human potential worldwide. A major goal of basic research on AUD is to understand the transition from alcohol use to alcohol dependence by revealing changes in neurotransmitter systems (Tabakoff and Hoffman, 2013). The Ɣ-aminobutyric acid type A (GABAA) receptors contribute to the behavioral effects of ethanol and play central roles in the short and long term effects of alcohol (Kumar et al., 2009). The function of GABAA receptors is modulated by potent endogenous neuroactive steroids that are produced in the brain, adrenal glands, and gonads (Gunn et al., 2015).
The GABAergic neuroactive steroids function as positive allosteric modulators of GABAA receptors, enhancing GABAergic function at nanomolar concentrations (Morrow et al., 1987). GABAergic neuroactive steroids act through specific binding sites on the α subunits to increase GABAA receptor activity to produce effects pharmacologically similar to ethanol (Hosie et al., 2006). Systemic administration of GABAergic neuroactive steroids exerts pharmacological responses including anxiolytic, antidepressant, anticonvulsant, sedative, and anesthetic effects in animal models and human studies that reflect their actions on GABAA receptors (Belelli et al., 1989, Bitran et al., 1991, Khisti et al., 2000, Hogskilde et al., 1987).
The effects of ethanol involve several GABAergic mechanisms that contribute to many of its behavioral actions. (3α,5α)-3-hydoxy-pregnane-20-one (3α,5α-THP or allopregnanolone) is one of the most potent GABAergic steroids and has been frequently studied in alcohol research. Ethanol increases de novo synthesis of 3α,5α-THP in rat brain (VanDoren et al., 2000), likely contributing to the changes in the GABAA receptor function. Moreover, neuroactive steroids contribute to alcohol sensitivity and may influence the risk of AUD (Morrow et al., 2006, Porcu and Morrow, 2014). Increasing evidence suggests that 3α,5α-THP, like ethanol, plays a role in modulating plastic changes on GABAA receptors (Concas et al., 1998, Follesa et al., 2000, Follesa et al., 2006). Taken together, brain 3α,5α-THP levels are involved in various mechanisms of alcohol related effects in central nervous system.
Recent studies elucidated the connection between chronic ethanol exposure and brain 3α,5α-THP levels. Studies in animals have shown that 17 days of voluntary ethanol drinking under limited access conditions produced a 1.65 fold increase in whole brain 3α,5α-THP concentration in male C57BL/6J mice, but not in the female mice (Finn et al., 2004). High levels of ethanol consumed in liquid diet for 14 days produced a small decrease in cortical 3α,5α-THP levels in male ethanol-dependent rats, but not in the female rats, while cortical 3α,5α-THP levels did not differ in ethanol withdrawn rats vs. controls (Janis et al., 1998). Similarly, 2 month daily intragastric ethanol administration and withdrawal decreased 3α,5α-THP content measured by gas chromatography-mass spectroscopy in cerebral cortex and hippocampus in Sprague Dawley rats (Cagetti et al., 2004). Recent studies using immunohistochemistry showed that intermittent ethanol exposure and withdrawal over 4 weeks decreases cellular 3α,5α-THP levels in the medial prefrontal cortex, ventral tegmental area, nucleus accumbens core, lateral amygdala, central nucleus of the amygdala, and dorsolateral striatum in C57BL/6J mice, but increases 3α,5α-THP levels in the CA3 pyramidal cell layer of the hippocampus, and does not alter 3α,5α-THP levels in several other limbic brain regions (Maldonado-Devincci et al., 2014). In cynomolgus monkeys, 12 months of voluntary ethanol consumption decreases 3α,5α-THP levels in lateral and basolateral amygdala where 3α,5α-THP immunoreactivity densities were negatively correlated with average daily drinking (Beattie et al., 2015). These animal studies have shown that ethanol produces divergent brain and cell specific changes in 3α,5α-THP concentrations which can differ across species.
The role 3α,5α-THP in alcohol actions in humans have been examined in only a few studies, primarily sampling human blood. Human male and female adolescents seen in the emergency room for alcohol intoxication had elevated 3α,5α-THP plasma levels (Torres and Ortega, 2003, Torres and Ortega, 2004). In contrast, ingestion of 3 standard drinks over 1 hour failed to alter plasma 3α,5α-THP or other GABAergic neurosteroid levels in laboratory settings (Holdstock et al., 2006, Porcu et al., 2010, Pierucci-Lagha et al., 2006), suggesting that alcohol dose might be a key factor. In addition, pretreatment with the neurosteroidogenic enzyme inhibitors finasteride and dutasteride reduced the subjective effects of acute alcohol administration in humans (Pierucci-Lagha et al., 2005, Covault et al., 2014). Studies on human AUD patients have shown that plasma 3α,5α-THP levels are decreased in early alcohol withdrawal and returned to normal levels upon recovery (Romeo et al., 1996). Recently, a study sampling postmortem human tissue has shown that pregnenolone (a precursor to 3α,5α-THP) and dehydroepiandrosterone (DHEA, a precursor of the GABAergic steroid androsterone) levels were higher in several limbic brain areas of AUD patients compared to non-alcoholic controls, although testosterone levels were similar in the same regions across groups (Karkkainen et al., 2016). To our knowledge, this is the first study of brain 3α,5α-THP levels in human AUD patients.
The ventral tegmental area (VTA) is part of the mesolimbic pathway, and is strongly implicated in ethanol reinforcement (McBride et al., 1999). Recently, our lab has shown that viral vector mediated over-expression of the P450scc enzyme, the rate limiting mitochondrial enzyme initiating steroidogenesis, causes an increase in 3α,5α-THP immunoreactivity in the VTA and reduces operant ethanol self-administration in rats (Cook et al., 2014b), suggesting that 3α,5α-THP levels in the VTA may regulate ethanol drinking. Furthermore, chronic intermittent ethanol exposure reduced 3α,5α-THP immunoreactivity in VTA of C57BL/6J mice (Maldonado-Devincci et al., 2014). The substantia nigra is part of the midbrain dopamine system, and provides dopaminergic tone necessary for voluntary movement (Bissonette and Roesch, 2016). Pars medialis substantia nigra (SNM) is located in the ventromedial aspect of the substantia nigra pars compacta (SNC) (Halliday, 2012), next to the VTA. Recent findings suggest that dopaminergic neurons in the SNC may also play a role in reward prediction processes (Matsumoto and Hikosaka, 2009) and ethanol consumption (Kim et al., 2015). Dopaminergic neurons in the ventromedial region in the SNC and VTA primarily project to the ventral striatum and transfer information related to reward values (Matsumoto and Hikosaka, 2009, Nomoto et al., 2010). The amygdala is a brain region that affects multiple aspects of human behavior, including anxiety (Gilpin et al., 2015) and alcohol seeking (Chaudhri et al., 2013). Long-term ethanol exposure has been shown to alter 3α,5α-THP levels in the amygdala subregions studied in mice (Maldonado-Devincci et al., 2014) and monkeys (Beattie et al., 2015). The present study examined the effects of AUD on 3α,5α-THP levels in the VTA, SNM and amygdala of human postmortem brains.
Materials and Methods
Subjects
Formalin fixed paraffin embedded (FFPE) human postmortem brain sections from 38 AUD patients and 27 control cases were obtained from the New South Wales Brain Tissue Resource Center (NSWBTRC) at the University of Sydney in Australia. Clinical information regarding subjects was provided by NSWBTRC, including age, sex, cause of death, smoking history, liver pathology, lifetime alcohol consumption (kg), average daily ethanol intake (g), and total drinking years. Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994) criteria were used to diagnose patients for AUD. Comorbidity with neurologic, psychiatric, and neurodegenerative disorders were the exclusion criteria for this study to eliminate confounding factors. Sex, age, alcohol consumption rate, postmortem interval, and lengths of the tissue fixation time in AUD patients and control subjects are reported in Table 1. Cardiovascular and respiratory system related complications were the most common causes of death for the AUD patients (58%) and control cases (87%).
Table 1.
Patient Characteristics of Human Postmortem Brains
| Brain Region: | Ventral Tegmental Area | Lateral Amygdala | ||||
|---|---|---|---|---|---|---|
| Substantia Nigra Pars Medialis | Basolateral Amygdala | |||||
| Sex | Male | Female | Male | |||
| Group | Control | AUD | Control | AUD | Control | AUD |
| Number of Subjects | 12 | 24 | 15 | 14 | 13 | 23 |
| Age of Death | 56.6±3 | 56.0±2 | 56.5±3 | 59.8±3 | 57.8±4 | 56.7±2 |
| Mean Alcohol Consumption (g/day) | 15.3±5 | 234±30 | 8.8±3 | 206±25 | 15.9±5 | 212±29 |
| Total Drinking Years | 27.9±6 | 32.0±1 | 25.9±6 | 31.9±3 | 28.6±5 | 32.3±2 |
| Alcohol lifetime use (kg) | 191±90 | 2650±342 | 114±39 | 2253±370 | 228±96 | 2449±297 |
| Postmortem Interval (hours) | 32.9±3 | 35.0±3 | 28.9±3 | 32.5±5 | 32.4±4 | 36.9±3 |
| Time In Fixation (days) | 25.4±2 | 24.8±2 | 20.5±1 | 22.9±1 | 2479±362 | 2307±270 |
Values were reported as mean ± standard error of mean (SEM)
Pathological information regarding postmortem human samples was provided by NSWBTRC, including brain pH, post-mortem interval, and length of tissue fixation time (Table 1.). The mean brain pH was 6.5±0.1 for all groups. Length of tissue fixation time varied between brain regions that are included in the study. The average fixation time for VTA and SNM regions, which are located in human midbrain, averaged 23.5±1 days. However, human amygdala samples were stored in formalin for 6±1 years.
Effect of the liver status was examined to determine if liver pathology contributed to the effect of AUD on 3α,5α-THP immunoreactivity. Autopsy information of pathological changes were categorized as normal liver, congestion, mild or moderate steatosis (fatty liver), and cirrhosis. Among AUD patients, 55% of the subjects were complicated with liver cirrhosis, 40% of the subjects had congestion or mild to moderate steatosis, and only 5% of the subjects had normal liver. In comparison, 60% of the control cases had no liver pathology (normal liver), while 40% of the control cases had mild congestion or mild liver steatosis. None of the control subjects had liver cirrhosis.
Immunohistochemistry (IHC)
IHC was performed on FFPE brain slides using a procedure modified from rat brain (Cook et al., 2014a) to detect cellular 3α,5α-THP levels. Slides were deparaffinized with xylene, rehydrated with gradually decreasing ethanol concentrations, and rinsed in phosphate buffered saline (PBS) at pH 7.4. After rinsing, tissues were incubated in 0.6% H2O2 to block endogenous peroxidase activity. Antigen retrieval was performed using 10X Citra Plus buffer (BioGenex, Fremont, CA) at pH 6.0 at 100 °C for 30 min. This was followed by rinsing in PBS. Slides were then blocked for 1 hr with 10% rabbit serum (Vector Laboratories, Burlingame, CA) and incubated in the sheep affinity-purified anti-3α,5α-THP primary antibody (purchased from Dr. R.H. Purdy) in 1:2500 dilution for 48 hr at 4°C. Next, sections were rinsed in PBS, and followed by incubation in a rabbit anti-sheep biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 60 min. Avidin-biotin amplification was performed with Vectastain Elite ABC Kit (Vector Laboratories), and immunoreactivity was visualized with nickel enhanced 3,3’-diaminobenzidine (Sigma-Aldrich, St. Louis, MO) based on manufacturers’ recommended protocol. Tissue was then dehydrated with gradually increasing ethanol concentrations and cleaned with xylene. Sections were cover slipped with Cytoseal XYL (Richard-Allen Scientific, Kalamazoo, MI) mounting media and sealed.
Anatomical Delineation
The term “ventral tegmental area (VTA)” has been used to refer to a collective name for parabranchial pigmented nucleus (PBP), paranigral nucleus (PN), parapeduncular nucleus (PaP), interfascicular nucleus (IF), rostral linear nucleus of the raphe (RLi), and caudal linear nucleus of the raphe (CLi) (Halliday and Tork, 1986, Halliday, 2012). VTA is also described as a nucleus, which is found at the ventromedial aspect of the red nucleus and is situated medial to the PBP in the transverse plane (McRitchie et al., 1996, Halliday, 2012, Reyes et al., 2012). We adopted this definition of the VTA nucleus term for this study (see Figure 1).
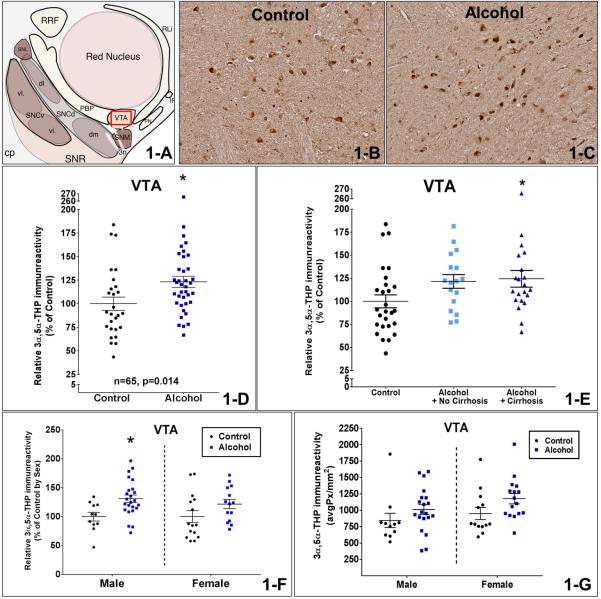
Figure 1.
Figure 1-A is a schematic diagram of the intermediate midbrain in the rostro-caudal transverse axis. Figures 1-B and C show representative photomicrographs (10X) of 3α,5α-THP immunostaining in the VTA of control and AUD patients. The red box in Figure 1-A shows the VTA region that is represented in the photos. Figure 1-D shows the comparison between controls and AUD patients. There was a 23.2±9% increase in 3α,5α-THP immunoreactivity of AUD patients compared to controls (p=0.014) in the VTA. Figure 1-E shows the comparison based on the liver pathology as AUD with cirrhosis, AUD with no cirrhosis, and controls in the VTA. There was 24.5±10% increase in 3α,5α-THP immunostaining from AUD subjects with cirrhosis compared to controls (p<0.05). Figure 1-F shows the comparison between controls and AUD patients independently for each sex. Male AUD patients had 31.0±11% higher 3α,5α-THP levels than male controls in the VTA (p<0.01) after the data was normalized to % of control values by sex. Figure 1-G shows the evaluation of the sex-related differences by comparing actual pixel density levels of 3α,5α-THP between males and females in the VTA. There was no difference in 3α,5α-THP immunostaining between male and female subjects (p>0.05) in the VTA. ANOVA analysis indicated no significant interaction between the effects of AUD and sex on 3α,5α-THP immunoreactivity (p=0.73) and the main effect analysis showed that AUD produced an increase in 3α,5α-THP immunoreactivity (p=0.032) in the VTA.
The ventromedial aspect of the substantia nigra, known as the substantia nigra pars medialis (SNM), is located ventrolateral to the red nucleus and is found next to the VTA in the rostro-caudal transverse sections (McRitchie et al., 1996, Halliday, 2012). VTA and SNM were sectioned in the transverse plane and examined simultaneously in the midbrain. Human amygdala slides were sectioned and examined in the coronal plane. Atlases of the human brain were used to identify anatomical boundaries and denomination of the sub regions of the amygdaloid complex (Mai et al., 2008).
IHC analyses
Brain region immunoreactivity was visualized with an Olympus CX51 light microscope (Olympus America, Center Valley, PA, USA); images were captured with a digital camera (Regita model; QImaging, Burnaby, BC, Canada) and analyzed using Bioquant (Nashville, TN, USA) image analysis to obtain linear integrated optical density. The microscope, camera, and software were background corrected to eliminate non-specific labeling and normalized to preset light levels to ensure fidelity of the data acquisition. Immunoreactivity was quantified by pixel density/mm2 from a circumscribed field, delineated as a brain region, divided by the area of the region in square millimeters. Additionally, positive cell count measurements were calculated from a defined region, divided by the area of the region in square millimeters (cell count/mm2). Cell count analysis yielded similar results to pixel density/mm2 analysis, suggesting that changes in the number of positive cells are a major component of pixel density analysis (data not shown). However, cell count data can be inaccurate when cells overlap in the field of analysis, usually leading to underestimation of the number of cells. Therefore, the results were only reported as pixel density/mm2 to maximize accuracy and avoid duplication of the data. Data from 4 sequential sections per subject from a single hemisphere were used to average a single value per region. Three comparable regions in lateral amygdala, 2 comparable regions in basolateral amygdala, and 1 comparable region in VTA and SNM were analyzed under 4x magnification to average a single value per subject. The experimenter was blind to the condition of each subject when analyses were conducted.
Double Immunofluorescent Labeling of 3α,5α-THP with NeuN and TH
Double immunofluorescent labeling and confocal microscopy was performed on FFPE brain slides to detect cell types that exhibit 3α,5α-THP positive immunostaining. After deparaffinization and rehydration, antigen retrieval was performed using 10X Citra Plus buffer at pH 6.0 at 100 °C for 15 min. Slides were then rinsed in PBS and blocked for 1 hr with 10% donkey serum (Jackson Immunoresearch Labs Inc., West Grove, PA) and incubated in primary antibody for cell type specific markers: TH (1:1000; ImmunoStar) or NeuN (1:1000, (D4G40) XP, Cell Signaling Technologies) for 24 hr at 4°C. Next, slides were rinsed, blocked, and incubated with anti-3α,5α-THP primary antibody for 48 hr at 4°C. Then, sections were rinsed and incubated with secondary antibody (Alexa Fluor 488 for 3α,5α-THP visualization and Alexa Fluor 594 for cell-type specific markers; Life Technologies). Immunofluorescence was visualized using a Leica SP2 laser scanning confocal microscope and computer software.
3α,5α-THP Antibody
The anti-3α,5α-THP antibody used for these studies was previously described (Cook et al., 2014). We observed cross reactivity with 3α-hydroxy-4-pregnen-20-one (3α-HP; 41±0.14%), (3α,5β)-3-hydroxypregnan-20-one (3α,5β-THP; 22±0.43%), progesterone (14±1.95%), 3α,5α-THDOC (11±0.29%), and pregnenolone (9±1.61%). In the present study, we tested for cross reactivity with the GABAergic metabolites of testosterone and DHEA by radioimmunoassay (RIA). Standards of 3α,5α-THP, 3α,5α-androstandiol, 3α,5β-androstandiol, 3α,5α-androsterone, and 3α,5β-androsterone were diluted in 95% EtOH at an initial concentration of 100 μg/ml. RIAs were repeated twice for each compound, following previously described methods (Janis et al., 1998, Cook et al., 2014a). Briefly, 5 μl of each concentration of the steroids (14 concentrations, 40 – 0.0049 ng/ml) was mixed with 10,000 counts per minute of [3H]3α,5α-THP, and a 1:500 dilution of the affinity purified antiserum. Unbound [3H]3α,5α-THP was removed by centrifugation after adding dextran-coated charcoal. The supernatant was mixed with Ecoscint H (National Diagnostics, Atlanta, GA) and [3H]3α,5α-THP was measured in a scintillation counter. The resulting curves were analyzed using a one-site competition model (Prism; GraphPad Software, La Jolla, CA) for EC50 values. We observed minimal cross-reactivity with 3α,5α-androstandiol (1.13%), 3α,5β-androstandiol (1.3E-06%), 3α,5α-androsterone (0.85%), and 3α,5β-androsterone (0.66%).
Statistical analysis
Raw pixel densities (average pixels/mm2) were analyzed in amygdala sections and used for direct comparison. Due to large sample sizes of human midbrain sections (VTA and SNM), immunostaining was performed in sequential assays. Data from each assay were transformed to percent of control (individual mean/[control group mean]*100) values, and compared statistically between groups. Student's t tests (Prism; Graphpad software) were used to compare 3α,5α-THP levels from control subjects and AUD patients. Pearson's correlation was used to determine the association between lifetime total alcohol drinking (kg) and mean alcohol consumption per day with 3α,5α-THP immunoreactivity. Analysis of variance (ANOVA) was used to determine the effects of sex, liver status, and smoking status on 3α,5α-THP immunoreactivity. Pearson's correlation and linear regression analysis were performed to determine the effect of age, postmortem interval, and the length of time in fixation with 3α,5α-THP immunoreactivity to detect potential confounding factors. All values were reported as mean ± standard error of mean (SEM) and significance were defined at p ≤ 0.05. A single investigator conducted all IHC and statistical analysis.
Results
Human Midbrain Samples
ANOVA analyses indicated no significant differences in age (F(1,63)= 0.28, p= 0.98), postmortem interval (PMI) (F(1,63)= 1.58, p= 0.31) or time in fixation (TIF) (F(1,63)= 0.52, p= 0.94) between controls and AUD patients in the VTA and SNM regions. There were no significant correlations between in 3α,5α-THP immunoreactivity and age, PMI, or TIF of subjects in the VTA and SNM regions (Pearson's test, p>0.05). It is notable that the average 3α,5α-THP immunoreactivity was 5.8 times higher in the SNM region than the VTA region (p<0.0001).
3α,5α-THP Levels in Human VTA of AUD Patients Differed from Control Subjects
Cellular 3α,5α-THP levels between AUD patients and control subjects were examined in the VTA. There was a 23.2±9% increase in 3α,5α-THP immunoreactivity of AUD patients compared to controls (Student's t test, t(63)= 2.517, p= 0.014, Figure 1-D) in the VTA. However, correlations between 3α,5α-THP immunoreactivity and total lifetime alcohol drinking (kg) (r = −0.06, n= 38, p= 0.74), mean daily alcohol consumption (gram) (r = −0.10, n= 36, p= 0.55), or total drinking years (r= 0.17, n=38, p=0.29) were not significant among AUD patients in the VTA by Pearson tests.
Next, the effects of AUD were evaluated independently in each sex, such that male or female AUD patients were directly compared to their respective same sex controls, after the data was normalized to % control values. Male AUD patients had 31.0±11% higher 3α,5α-THP levels than male controls in the VTA (Student's t test, t(33)=2.87, p<0.01, Figure 1-F), but similar differences in female controls and AUD patients (21.8±13%) did not reach significance (p>0.05) in the VTA. To examine the possibility of sex differences, male and female subjects were reexamined in the same IHC assay, comparing actual pixel density levels of 3α,5α-THP and the related data was transformed as % of male controls (Figure 1-G). In this experiment, there was no difference in 3α,5α-THP immunoreactivity between male and female subjects (p>0.05). Two-way ANOVA with sex and AUD as factors indicated no interaction between the effects of AUD and sex on 3α,5α-THP immunoreactivity (F(1, 56) = 0.12, p= 0.73). The main effect analysis showed that AUD produced an increase in 3α,5α-THP immunoreactivity (F(1, 56) = 4.85, p= 0.032), while sex had no significant effect on 3α,5α-THP immunoreactivity (p= 0.13) in the VTA.
Sex-Specific Effect of AUD on 3α,5α-THP Immunoreactivity in Substantia Nigra Pars Medialis
No significant difference in 3α,5α-THP immunoreactivity of AUD patients compared to controls was found in the SNM (Student's t test, t(61)=1.506, p=0.14, Figure 2-D). Among AUD patients, correlations between 3α,5α-THP immunoreactivity and total lifetime alcohol drinking (kg) (r = 0.09, n =36, p= 0.64), mean daily alcohol consumption (gram) (r =0.17, n=36, p = 0.32), or total drinking years (r=0.05, n=36, p=0.77) were not significant in the SNM by Pearson tests.
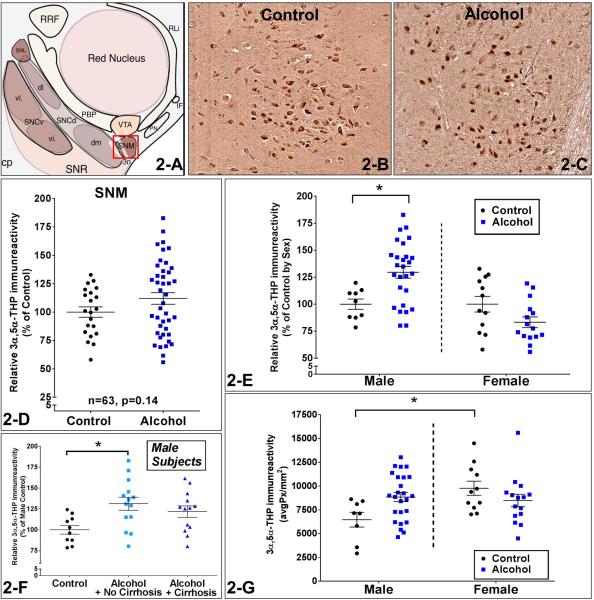
Figure 2.
Figure 2-A is a schematic diagram of the intermediate midbrain in the rostro-caudal transverse axis. Figures 2-B and C show representative photomicrographs (10X) of 3α,5α-THP immunostaining in substantia nigra pars medialis (SNM) of controls and AUD patients. The red box in Figure 2-A shows the SNM region that is represented in the photos. Figure 2-D shows the comparison between controls and AUD patients. No significant difference in 3α,5α-THP immunoreactivity of AUD patients compared to controls was found in the SNM (p=0.14). Figure 2-E shows the comparison between controls and alcoholic subjects independently for each sex after the data was normalized to % of control values. Male AUD patients had 29.6±10% higher 3α,5α-THP levels than male controls in the SNM (p<0.01). Figure 2-G shows the evaluation of the sex-related differences by comparing actual pixel density levels of 3α,5α-THP between males and females in the SNM. Female control subjects had 51.1±18% higher 3α,5α-THP immunoreactivity than male controls in the SNM (p<0.05). There was a statistically significant interaction between the effects of AUD and sex on 3α,5α-THP immunoreactivity (p=0.01) and the main effect analysis showed that sex had a significant effect on 3α,5α-THP immunoreactivity (p=0.038). Figure 2-F shows the comparison based on the liver pathology in the male subjects as AUD with cirrhosis, AUD with no cirrhosis, and controls in the SNM. There was a 31.2±9% increase in 3α,5α-THP immunoreactivity of male non-cirrhotic AUD patients compared to the male controls (p=0.02).
Sex-specific effects of AUD were examined as described above. First, the effects of AUD were evaluated independently in each sex. Male AUD patients had 29.6±10% higher 3α,5α-THP levels than male controls in the SNM (Student's t test, t(34)=2.967, p<0.01, Figure 2-E), while there was no significant difference between female AUD patients and female controls (p>0.05), despite the observation that female AUD patients averaged 16.7±9% lower 3α,5α-THP levels than female controls in the SNM. We also compared actual pixel density levels of 3α,5α-THP between males and females in a separate IHC experiment (Figure 2-G). Female control subjects had 51.1±18% higher 3α,5α-THP immunoreactivity than male controls in the SNM (p<0.05).
There was a statistically significant interaction between the effects of AUD and sex on 3α,5α-THP immunoreactivity (F(1, 55) = 7.04, p= 0.01) and the main effect analysis showed that sex had a significant effect on 3α,5α-THP immunoreactivity (F(1, 55) = 4.48, p= 0.038). We conclude 3α,5α-THP levels increased only in the male AUD patients compared to male controls and sex is an influencing factor on 3α,5α-THP levels in the SNM. .
Potential confounding factors related to the 51.1±18% increase in the female controls compared to male controls in the SNM were further explored. However, we found no significant difference in age, PMI, TIF, brain pH, mean daily alcohol consumption (gram), lifetime alcohol drinking (kg), total drinking years, and pack-years smoking history between female controls vs. male controls (p>0.05).
The Effect of Comorbidity with Liver Cirrhosis on 3α,5α-THP Immunoreactivity in AUD
The subjects were compared based on the liver pathology as AUD with cirrhosis (n=21), AUD with no cirrhosis (n=17), and controls (n=27) (Figure 1-E) by ANOVA and post-hoc analyses in the VTA. There was 24.5±10% increase in 3α,5α-THP immunostaining for AUD subjects with cirrhosis compared to controls (F(2,62)=3.15, p<0.05). The difference between AUD subjects with no cirrhosis and controls was not significant despite the observation that non-cirrhotic AUD patients had 21.6±11% higher 3α,5α-THP levels in the VTA (p>0.05). There was no difference between 3α,5α-THP immunoreactivity of cirrhotic AUD patients and non-cirrhotic AUD patients (p>0.05) in the VTA. Thus, 3α,5α-THP levels in the VTA of the non-cirrhotic and cirrhotic AUD patients were likely similarly affected.
Since the effects of AUD on 3α,5α-THP immunoreactivity in SNM were only observed in the male subjects, the role of liver pathology was examined in three groups of male subjects: AUD with cirrhosis (n=13), AUD with no cirrhosis (n=14), and controls (n=10) (Figure 2-F). There was a 31.2±9% increase in 3α,5α-THP immunoreactivity of male non-cirrhotic AUD patients compared to the male controls (F(2,34)=4.76, p= 0.02). The difference between male AUD with cirrhosis and male controls was not significant despite the observation that male cirrhotic-AUD patients had 21.8±10% higher 3α,5α-THP levels in the SNM. There was no difference between 3α,5α-THP immunoreactivity of cirrhotic AUD patients and non-cirrhotic AUD patients (p>0.05) in the SNM. Thus, 3α,5α-THP levels in the SNM of the non-cirrhotic and cirrhotic AUD patients were likely affected similarly.
We examined the role of smoking history in all subjects, since 66% of the controls and 75% of the AUD patients were reported as smokers. Average cigarette smoking was 33.3±3 pack-years in all cases with no significant difference between controls and AUD patients (p= 0.35). There was also no significant correlation between pack-years smoking history and 3α,5α-THP immunoreactivity in either VTA or SNM regions (Pearson's test, p>0.05).
3α,5α-THP co-localizes with TH and NeuN in the VTA and SNM
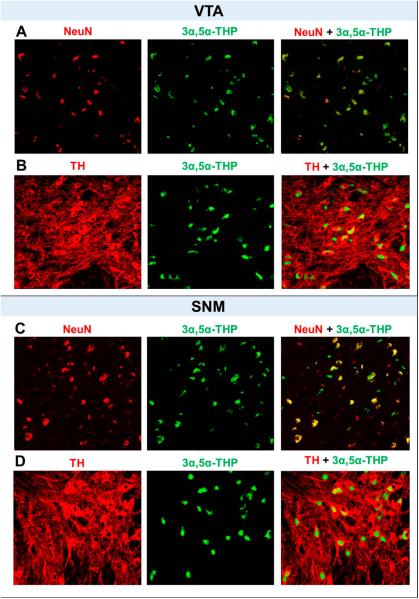
To identify cell types in which 3α,5α-THP is localized in the VTA and SNM, we conducted double immunofluorescent labeling of 3α,5α-THP with NeuN and TH in human control subjects not included in the experiments above. We found that 3α,5α-THP shows a very high rate of co-localization with NeuN in the VTA and SNM (Figure 3 A and C). This finding suggests that 3α,5α-THP is primarily localized in neurons in the VTA and SNM. We also observed that 3α,5α-THP was localized in TH positive neurons to a large extent in the VTA and SNM (Figure 3 B and D) suggesting that 3α,5α-THP is largely, but not exclusively, found in the dopaminergic neurons of the VTA and SNM.
Figure 3.
Dual immunolabeling with NeuN (red) or TH (red) with 3α,5α-THP (green) in the VTA (A and B). Dual immunolabeling with NeuN (red) or TH (red) with 3α,5α-THP (green) in the SNM (C and D). Confocal scanning showed that 3α,5α-THP co-localizes with NeuN positive and TH positive neurons in the VTA and SNM of postmortem human brain sections.
Time in fixation (TIF) and Age were Negatively Related to 3α,5α-THP Immunoreactivity in Human Amygdala Regions
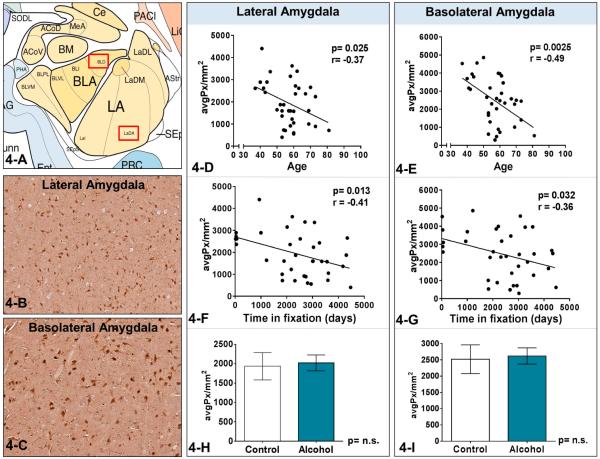
ANOVA analyses indicated no significant differences in PMI (F(1,34)= 0.017, p=0.99), or age (F(1,34)= 0.004, p=0.99) or TIF (F(1,34)= 0.65, p=0.88) between controls and AUD patients in the lateral and basolateral amygdala. Pearson's test was used to assess correlations between in 3α,5α-THP immunoreactivity and PMI (days), age (year), or TIF (days) in the lateral and basolateral amygdala. There was no correlation between in 3α,5α-THP immunoreactivity and PMI in lateral or basolateral amygdala (p>0.05). However, 3α,5α-THP levels were negatively related to the age of subjects in lateral amygdala (r = −0.37, p=0.025, n=36) and basolateral amygdala (r = −0.49, p=0.0025, n=36) (Figure 4-D and 4-E). 3α,5α-THP levels were also negatively related to the time in fixation of subjects in lateral amygdala (r = −0.41, p=0.013, n=36) and basolateral amygdala (r = −0.36, p=0.032, n=36) (Figure 4-G and 4-F). These factors confound the analysis of potential differences between controls and AUD patients. Nonetheless, we analyzed the lateral and basolateral amygdala regions to compare cellular 3α,5α-THP immunoreactivity between controls and AUD patients. There was no significant difference in 3α,5α-THP immunoreactivity between controls and AUD in the lateral amygdala (t(31)=0.23, p=0.82, Figure 4-H) and basolateral amygdala (t(31)=0.22, p=0.83, Figure 4-I).
Figure 4.
Figure 4-A is a schematic diagram of the amygdala in the coronal plane. Figures 4-B and C show representative photomicrographs (10X) of 3α,5α-THP immunostaining in the lateral and basolateral amygdala. The red boxes in Figure 4-A show the regions that are represented in the photos. Figure 4-D and 4-E indicate that 3α,5α-THP levels were negatively related to the age of subjects in lateral amygdala (Pearson's r value= −0.37, p=0.025, n=36) and basolateral amygdala (Pearson's r value= −0.49, p=0.0025, n=36). Figure 4-F and 4-G show that 3α,5α-THP levels were negatively related to the time in fixation of samples in lateral amygdala (Pearson's r value= −0.41, p=0.013, n=36) and basolateral amygdala (Pearson's r value= −0.36, p=0.032, n=36).
Discussion
The present set of experiments show that cellular 3α,5α-THP levels are 23.2±9% higher in the VTA of human AUD patients compared to the age matched controls. The difference in 3α,5α-THP levels in the VTA of AUD patients was driven by males. There was not a significant difference among female subjects, although the absolute difference was similar to male subjects. The lack of effect in females may be due to higher variability in 3α,5α-THP levels of female control subjects or the smaller sample size. Unfortunately, additional samples were not available for analysis. Likewise, 3α,5α-THP levels were significantly higher in the SNM of male AUD patients compared to the male controls, while there was no difference among female AUD patients compared to female controls.
Dual label confocal microscopy revealed that cellular 3α,5α-THP expression was primarily localized in TH positive dopaminergic neurons in the VTA and SNM. This finding is consistent with our previous observation in rat brain (Cook et al., 2014b). In general, increases in cellular 3α,5α-THP levels are associated with an enhancement in GABAergic tone, resulting in a reduction in the neuronal excitability (Akk et al., 2005, Sanna et al., 2004). VTA dopaminergic activity increases after acute ethanol administration that is associated with the rewarding effect of ethanol and increased dopamine release within nucleus accumbens. Furthermore, withdrawal from chronic ethanol exposure reverses this effect and leads to a decrease in the activity of VTA dopaminergic neurons (Morikawa and Morrisett, 2010) that could be related to an increase in 3α,5α-THP levels in VTA dopamine neurons. It is well known that the last exposure time to ethanol is an important determinant of the activity of VTA dopaminergic neurons. Unfortunately, no information was available related to the interval between the last exposure to alcohol and the death of the subjects. Thus, we are unable to report the alcohol status immediately before death.
However, since the most common cause of death was chronic cardio-respiratory system failure, not accident or suicide, variations in drinking before death were likely due to hospitalizations or disease related impairments. Nonetheless, the increase in 3α,5α-THP levels may contribute to adaptive responses in the dopaminergic VTA neurons after chronic ethanol exposure. However, we cannot eliminate the probability that an increase in local 3α,5α-THP levels also affects the GABAergic and glutamatergic neurons in the VTA. While little is known about the VTA micro-circuitry connections and sub-regions in human brain, further experimentation will be needed to understand how local 3α,5α-THP alterations in the VTA affect human mesolimbic activity and drinking behavior.
These results differ from studies in animal models of AUD. We have previously shown a decrease in 3α,5α-THP levels in the VTA of C57BL/6J mice after chronic intermittent ethanol exposure and withdrawal (Maldonado-Devincci et al., 2014). In that study, we examined coronal sections of VTA at the level of bregma −3.08, while the human study examined VTA in the transverse plane at the level of the red nucleus. Therefore, we cannot rule out the possibility that differences in the VTA subregions investigated contribute to the different results in the two studies. Other studies reported a similar increase in whole brain 3α,5α-THP levels after voluntary ethanol consumption for 17 days in C57BL/6J mice (Finn et al., 2004), decreases in cortical and hippocampal 3α,5α-THP concentrations following chronic EtOH exposure in rats (Janis et al., 1998, Cagetti et al., 2004), and decreases in amygdala subregions of non-human primates after one year of ethanol consumption (Beattie et al., 2015). Possible explanations for the discrepancy between human AUD patients and rodent models of chronic alcohol exposure include influences such as the age of the subjects (humans: age 57; rodents: young adults), duration of alcohol exposure (humans: ~30 yr; rodents: 2-6 weeks), and co-occurring liver disease in humans that is quite rare in rodent models of chronic alcohol exposure (Tsukamoto et al., 1995). Furthermore, studies in rat models used radioimmunoassay (Janis et al., 1998) or gas chromatography-mass spectroscopy (Cagetti et al., 2004) to measure 3α,5α-THP levels, while levels in human VTA and SNM were determined by immunohistochemistry. Another notable difference in the present study is the presence of ethanol exposure in human control subjects. Social drinkers who consumed 1-2 drinks per day were analyzed as control subjects in this study. On the other hand, alcohol naïve animals were used as the control group in all rodent studies. Therefore, differences between animal models and human studies should be taken into consideration.
Increases in 3α,5α-THP levels in the VTA and partially in the SNM (only among male subjects) are concordant with a recent post-mortem brain study examining steroid levels in human AUD patients. In the study, pregnenolone (a precursor to 3α,5α-THP) and dehydroepiandrosterone (a precursor of the GABAergic neuroactive steroid, androsterone) levels were higher in most limbic brain regions in AUD patients, compared to non-alcoholic controls (Karkkainen et al., 2016). Together, these findings may indicate a global increase in neurosteroid content in the brains of human AUD patients. Further studies are needed to test this hypothesis.
Potential cross-reactivity of anti-3α,5α-THP antibody with other neurosteroids should be taken into consideration. We have previously observed a 9±1.6% cross reactivity of anti-3α,5α-THP antibody with pregnenolone (Cook et al., 2014a) which may contribute to the similar outcomes with the recent post-mortem human study (Karkkainen et al., 2016). Cross-reactivities between anti-3α,5α-THP antibody and other neurosteroids such as 3α-HP or 3α,5β-THP has been also discussed in the previous work (Cook et al., 2014a). Additionally in this study, we detected negligible cross-reactivity between anti-3α,5α-THP antibody with 3α-reduced androstanes (see Materials and Methods) suggesting that the interactions with anti-3α,5α-THP and 3α-reduced androstanes are minimal.
The difference in the ages of human subjects vs. rodent studies deserves further consideration. We examined human AUD patients at an average age of 57, which would correspond to an 18+ month old rat (Sengupta, 2013). In comparison, studies investigating the effect of chronic ethanol exposure on brain 3α,5α-THP levels have primarily been conducted in 2 to 4 month old young adult rodents (Janis et al., 1998, Cagetti et al., 2004, Maldonado-Devincci et al., 2014) leaving gaps in our knowledge in the effect of alcohol on the neurosteroid levels in rodents vs. humans. Aged humans show differential GABAA receptor subunit expression (Kanaumi et al., 2006), have higher physiological sensitivity to alcohol (Menninger, 2002), and are more susceptible to the deficits associated with chronic AUD (Pfefferbaum et al., 1992). Further, aged animals show greater ethanol-induced memory impairment and exhibit fewer withdrawal symptoms compared to adult animals (Novier et al., 2016) suggesting that aged adult individuals have unique responses to chronic ethanol exposure. In this study, we also show that 3α,5α-THP concentrations are negatively correlated with age in amygdala regions, but not in the VTA and SNM regions. These finding may suggest that the 3α,5α-THP content in various brain areas are affected by the aging process differently.
Patients with a diagnosis for psychiatric, neurologic and neurodegenerative conditions were excluded from the study to eliminate confounding factors. However, AUD causes secondary medical conditions, including alcoholic liver disease. Cirrhosis is the final phase of alcoholic liver disease, characterized by irreversible hepatic tissue transformation. In our study, more than half of the AUD patients were complicated with liver cirrhosis, while the remaining AUD patients had milder and reversible manifestations of liver disease such as congestion or steatosis. Only 5% of the AUD patients showed no signs of liver disease. In our study, we found no significant difference in 3α,5α-THP brain levels of non-cirrhotic AUD patients compared to cirrhotic alcoholics, suggesting that AUD is the main factor in the observed increase in AUD patients. However, others have reported that in the majority of male individuals with alcoholic fatty liver change and alcoholic cirrhosis, 3α,5α-THP precursor progesterone plasma levels were above the upper limit of normal plasma progesterone concentration (Farthing et al., 1982). Moreover, increase in plasma 3α,5α-THP concentrations have been also shown in non-alcoholic liver injuries (Ahboucha et al., 2008). Besides, 3α,5α-THP is known to readily cross the blood brain barrier (Zhu et al., 2001) and 3α,5α-THP precursors are metabolized in the liver (Stanczyk, 2003). 3α,5α-THP levels were also significantly higher in the brain extracts from hepatic encephalopathy patients who died in hepatic coma (Ahboucha et al., 2006), but no difference was found between patients with uncomplicated liver disease and controls. Therefore, we conclude that 3α,5α-THP elevations in VTA are not substantially moderated by liver disease.
In the SNM, female control subjects averaged 51±18% higher brain 3α,5α-THP levels than male control subjects, but this effect was not observed in the VTA. In this study, we also compared VTA and SNM 3α,5α-THP levels between pre-menopausal women (<48yr) versus post-menopausal women (>55yr) by grouping them according to adjusted median age of natural menopause (51.7yr) in the Australian population (Do et al., 1998). However, comparisons of these two age groups were not significantly different in any brain region studied (data not shown). Other human studies show that average brain progesterone and 3α,5α-THP concentrations were significantly higher in the fertile women in the luteal phase of the menstrual cycle compared to their postmenopausal controls (Bixo et al., 1997), suggesting that females have higher basal 3α,5α-THP levels in the brain. Together, these findings may suggest that the basal 3α,5α-THP content in female brain is likely higher than male brain, but different brain areas may show greater sensitivity to sex-related effects.
Studies in amygdala sections of human AUD patients were confounded by the effects of fixation time in formalin. It is well know that immunohistochemistry is greatly influenced by the fixation time in formalin (Grillo et al., 2015). Accordingly, we found that 3α,5α-THP levels in the lateral and basolateral amygdala are negatively correlated with the fixation time in formalin. Average length of fixation time for human amygdala samples was more than 6 years, producing a challenge to measure 3α,5α-THP levels in amygdala regions. We also observed a negative correlation between age and 3α,5α-THP levels that may also confound the analysis of potential differences between control and AUD patients. On the other hand, VTA and SNM samples were fixed in formalin for less than a month on average, and there was no correlation between 3α,5α-THP levels and fixation time in the VTA or SNM. Therefore, antigenic epitopes in the VTA and SNM regions may be relatively preserved from any masking effect of formalin.
The observation that 3α,5α-THP levels were decreased with aging in amygdala, but not VTA or SNM, of humans subjects suggests regional variation in regulation of 3α,5α-THP synthesis or degradation. Animal studies examining the effect of aging on 3α,5α-THP brain levels have yielded inconsistent results. Brain 3α,5α-THP content showed a significant progressive decrease with age in Wistar rats (Bernardi et al., 1998) but showed no difference between 2 month old and 18 month old Sprague Dawley rats (Barbaccia et al., 1998). A similar decline has been observed in several rat brain regions that could be linked to sleep cycle alterations and cognitive impairment in aged rat populations (George et al., 2010). Taken together, 3α,5α-THP levels may show a region specific decrease in the brain with age that could contribute to physiological aging or age-related pathological conditions. Thus, future studies are required to expand our understanding of age-related differences.
The study had several limitations. First, relative values of immunoreactivity were determined via measuring the pixel density within a prescribed field over pre-established baseline levels. Therefore, values are relative and expressed as percent control or average pixel density, making it impossible to compare absolute 3α,5α-THP levels in the brain regions. Second, other GABAergic neuroactive steroid levels should be measured to understand the complete neurosteroid pattern in post-mortem human brain regions. Moreover, highly relevant brain regions including nucleus accumbens, hippocampus, prefrontal cortex, and anterior cingulate cortex should be investigated in order to compare data with rodent studies that examined more regions.
Alterations in 3α,5α-THP levels may contribute to adaptive responses in VTA neurons or the elevation of 3α,5α-THP levels may represent a biomarker of alcohol abuse. The differences between animal models and human studies should be considered to determine the physiological significance of elevated 3α,5α-THP levels in humans. In conclusion, cellular 3α,5α-THP levels in the VTA are increased in human AUD patients that are likely independent of sex and liver disease. Further studies are needed to determine the physiological significance of elevated 3α,5α-THP levels in humans.
Acknowledgements
Tissues were received from the New South Wales Brain Tissue Resource Centre at the University of Sydney, which is supported by the Schizophrenia Research Institute and National Institute of Alcohol Abuse and Alcoholism (NIH - NIAAA R28AA012725.)
Funding and Disclosures
This research was supported by the NIAAA INIA U01-AA020935 (ALM) and the UNC Bowles Center for Alcohol Studies.
Footnotes
The authors have no conflict of interest to declare.
References
- Ahboucha S, Butterworth RF, Pomier-Layrargues G, Vincent C, Hassoun Z, Baker GB. Neuroactive steroids and fatigue severity in patients with primary biliary cirrhosis and hepatitis C. Neurogastroenterol Motil. 2008;20:671–679. doi: 10.1111/j.1365-2982.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- Ahboucha S, Pomier-Layrargues G, Mamer O, Butterworth RF. Increased levels of pregnenolone and its neuroactive metabolite allopregnanolone in autopsied brain tissue from cirrhotic patients who died in hepatic coma. Neurochem Int. 2006;49:372–378. doi: 10.1016/j.neuint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Concas A, Serra M, Biggio G. Stress and neurosteroids in adult and aged rats. Exp Gerontol. 1998;33:697–712. doi: 10.1016/s0531-5565(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Beattie MC, Maldonado-Devincci AM, Porcu P, O'Buckley TK, Daunais JB, Grant KA, Morrow AL. Voluntary ethanol consumption reduces GABAergic neuroactive steroid (3alpha,5alpha)3-hydroxypregnan-20-one (3alpha,5alpha-THP) in the amygdala of the cynomolgus monkey. Addict Biol. 2015 doi: 10.1111/adb.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. European Journal of Pharmacology. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. European Journal of Endocrinology. 1998;138:316–321. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Roesch MR. Development and function of the midbrain dopamine system: what we know and what we need to. Genes Brain Behav. 2016;15:62–73. doi: 10.1111/gbb.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone, 5α-pregnan-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Research. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci. 2013;38:2751–2761. doi: 10.1111/ejn.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcoholism, Clinical and Experimental Research. 2014a;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Werner DF, Maldonado-Devincci AM, Leonard MN, Fisher KR, O'Buckley TK, Porcu P, McCown TJ, Besheer J, Hodge CW, Morrow AL. Overexpression of the steroidogenic enzyme cytochrome P450 side chain cleavage in the ventral tegmental area increases 3alpha,5alpha-THP and reduces long-term operant ethanol self-administration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014b;34:5824–5834. doi: 10.1523/JNEUROSCI.4733-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR. Dutasteride reduces alcohol's sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl) 2014;231:3609–3618. doi: 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KA, Treloar SA, Pandeya N, Purdie D, Green AC, Heath AC, Martin NG. Predictive factors of age at menopause in a large Australian twin study. Hum Biol. 1998;70:1073–1091. [PubMed] [Google Scholar]
- Farthing MJ, Green JR, Edwards CR, Dawson AM. Progesterone, prolactin, and gynaecomastia in men with liver disease. Gut. 1982;23:276–279. doi: 10.1136/gut.23.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Hormones and behavior. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- George O, Vallee M, Vitiello S, Le Moal M, Piazza PV, Mayo W. Low brain allopregnanolone levels mediate flattened circadian activity associated with memory impairments in aged rats. Biol Psychiatry. 2010;68:956–963. doi: 10.1016/j.biopsych.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo F, Pigozzi S, Ceriolo P, Calamaro P, Fiocca R, Mastracci L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol. 2015;144:93–99. doi: 10.1007/s00418-015-1316-4. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol. 2015;36:28–48. doi: 10.1016/j.yfrne.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G. Substantia nigra, ventral tegmental area and retrorubral fields. Elsevier Academic Press; 2012. [Google Scholar]
- Halliday GM, Tork I. Comparative anatomy of the ventromedial mesencephalic tegmentum in the rat, cat, monkey and human. J Comp Neurol. 1986;252:423–445. doi: 10.1002/cne.902520402. [DOI] [PubMed] [Google Scholar]
- Hogskilde S, Wagner J, Carl P, Sorensen MB. Anaesthetic properties of pregnanolone emulsion. A comparison with alphaxolone/alphadolone, propofol, thiopentone and midazolam in a rat model. Anaesthesia. 1987;42:1045–1050. doi: 10.1111/j.1365-2044.1987.tb05166.x. [DOI] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, De Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy-5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology. 2006;186:442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcoholism, clinical and experimental research. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Kanaumi T, Takashima S, Iwasaki H, Mitsudome A, Hirose S. Developmental changes in the expression of GABAA receptor alpha 1 and gamma 2 subunits in human temporal lobe, hippocampus and basal ganglia: An implication for consideration on age-related epilepsy. Epilepsy Res. 2006;71:47–53. doi: 10.1016/j.eplepsyres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Karkkainen O, Hakkinen MR, Auriola S, Kautiainen H, Tiihonen J, Storvik M. Increased steroid hormone dehydroepiandrosterone and pregnenolone levels in post-mortem brain samples of alcoholics. Alcohol (Fayetteville, N.Y.) 2016;52:63–70. doi: 10.1016/j.alcohol.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacology, Biochemistry and Behavior. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, Chen L, Ding JB. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350:102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. Third ed. Academic Press; San Diego: 2008. [Google Scholar]
- Maldonado-Devincci AM, Cook JB, O' Buckley TK, Morrow DH, McKinley RE, Lopez MF, Becker HC, Morrow AL. Chronic Intermittent Ethanol Exposure and Withdrawal Alters (3alpha,5alpha)-3-Hydroxy-Pregnan-20-One Immunostaining in Cortical and Limbic Brain Regions of C57BL/6J Mice. Alcoholism, clinical and experimental research. 2014;38:2561–2571. doi: 10.1111/acer.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Hardman CD, Halliday GM. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J Comp Neurol. 1996;364:121–150. doi: 10.1002/(SICI)1096-9861(19960101)364:1<121::AID-CNE11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Menninger JA. Assessment and treatment of alcoholism and substance-related disorders in the elderly. Bull Menninger Clin. 2002;66:166–183. doi: 10.1521/bumc.66.2.166.23364. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. International review of neurobiology. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Schultz W, Watanabe T, Sakagami M. Temporally extended dopamine responses to perceptually demanding reward-predictive stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:10692–10702. doi: 10.1523/JNEUROSCI.4828-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novier A, Ornelas LC, Diaz-Granados JL, Matthews DB. Differences in Behavioral Responding in Adult and Aged Rats Following Chronic Ethanol Exposure. Alcoholism, clinical and experimental research. 2016;40:1462–1472. doi: 10.1111/acer.13098. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism, clinical and experimental research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Porcu P, Morrow AL. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: relevance for human studies. Psychopharmacology (Berl) 2014;231:3257–3272. doi: 10.1007/s00213-014-3564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5α neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcoholism, clinical and experimental research. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Fu Y, Double K, Thompson L, Kirik D, Paxinos G, Halliday GM. GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. J Comp Neurol. 2012;520:2591–2607. doi: 10.1002/cne.23051. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharm. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P. The Laboratory Rat: Relating Its Age With Human's. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. The Journal of Neuroscience : the official journal of the Society for Neuroscience. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wang MD, Backstrom T, Wahlstrom G. Evaluation and comparison of the pharmacokinetic and pharmacodynamic properties of allopregnanolone and pregnanolone at induction of anaesthesia in the male rat. Br J Anaesth. 2001;86:403–412. doi: 10.1093/bja/86.3.403. [DOI] [PubMed] [Google Scholar]