Abstract
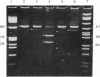
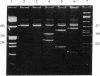
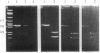
The expression of mRNAs coding for the alpha subunit of rat brain and rat heart sodium channels has been studied in adult and neonatal rat cerebral cortex using the reverse transcription-polymerase chain reaction (RT-PCR). Rat brain sodium channel subtype I, II, IIA, and III sequences were simultaneously amplified in the same PCR using a single oligonucleotide primer pair matched to all four subtype sequences. Identification of each subtype-specific product was inferred from the appearance of unique fragments when the product was digested with specific restriction enzymes. By using this RT-PCR method, products arising from mRNAs for all four brain sodium channel subtypes were identified in RNA extracted from adult rat cerebral cortex. The predominant component was type IIA with lesser levels of types I, II, and III. In contrast, the type II and IIA sequences were the predominant RT-PCR products in neonatal rat cortex, with slightly lower levels of type III and undetectable levels of type I. Thus, from neonate to adult, type II mRNA levels decrease relative to type IIA levels. Using a similar approach, we detected mRNA coding for the rat heart sodium channel in neonatal and adult rat cerebral cortex and in adult rat heart. These results reveal that mRNAs coding for the heart sodium channel and all four previously sequenced rat brain sodium channel subtypes are expressed in cerebral cortex and that type II and IIA channels may be differentially regulated during development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed C. M., Auld V. J., Lester H. A., Dunn R., Davidson N. Both sodium channel II and IIA alpha subunits are expressed in rat brain. Nucleic Acids Res. 1990 Oct 11;18(19):5907–5907. doi: 10.1093/nar/18.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld V. J., Goldin A. L., Krafte D. S., Catterall W. A., Lester H. A., Davidson N., Dunn R. J. A neutral amino acid change in segment IIS4 dramatically alters the gating properties of the voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1990 Jan;87(1):323–327. doi: 10.1073/pnas.87.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld V. J., Goldin A. L., Krafte D. S., Marshall J., Dunn J. M., Catterall W. A., Lester H. A., Davidson N., Dunn R. J. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron. 1988 Aug;1(6):449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Barchi R. L. Probing the molecular structure of the voltage-dependent sodium channel. Annu Rev Neurosci. 1988;11:455–495. doi: 10.1146/annurev.ne.11.030188.002323. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Beckh S., Noda M., Lübbert H., Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 1989 Dec 1;8(12):3611–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Chiu S. Y., Gray P. T., Ritchie J. M. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Chelly J., Montarras D., Pinset C., Berwald-Netter Y., Kaplan J. C., Kahn A. Quantitative estimation of minor mRNAs by cDNA-polymerase chain reaction. Application to dystrophin mRNA in cultured myogenic and brain cells. Eur J Biochem. 1990 Feb 14;187(3):691–698. doi: 10.1111/j.1432-1033.1990.tb15355.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cribbs L. L., Satin J., Fozzard H. A., Rogart R. B. Functional expression of the rat heart I Na+ channel isoform. Demonstration of properties characteristic of native cardiac Na+ channels. FEBS Lett. 1990 Nov 26;275(1-2):195–200. doi: 10.1016/0014-5793(90)81470-9. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Sherman S. J., Catterall W. A. Voltage clamp analysis of tetrodotoxin-sensitive and -insensitive sodium channels in rat muscle cells developing in vitro. J Neurosci. 1985 Sep;5(9):2559–2564. doi: 10.1523/JNEUROSCI.05-09-02559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Merrick D., Auld V., Dunn R., Goldin A. L., Davidson N., Catterall W. A. Tissue-specific expression of the RI and RII sodium channel subtypes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8682–8686. doi: 10.1073/pnas.84.23.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T., Chiu S. Y., Bevan S., Ritchie J. M. Ion channels in rabbit cultured fibroblasts. Proc R Soc Lond B Biol Sci. 1986 Feb 22;227(1246):1–16. doi: 10.1098/rspb.1986.0005. [DOI] [PubMed] [Google Scholar]
- Haimovich B., Tanaka J. C., Barchi R. L. Developmental appearance of sodium channel subtypes in rat skeletal muscle cultures. J Neurochem. 1986 Oct;47(4):1148–1153. doi: 10.1111/j.1471-4159.1986.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Kallen R. G., Sheng Z. H., Yang J., Chen L. Q., Rogart R. B., Barchi R. L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990 Feb;4(2):233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Koos R. D., Olson C. E. Expression of basic fibroblast growth factor in the rat ovary: detection of mRNA using reverse transcription-polymerase chain reaction amplification. Mol Endocrinol. 1989 Dec;3(12):2041–2048. doi: 10.1210/mend-3-12-2041. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Suzuki H., Numa S., Stühmer W. A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett. 1989 Dec 18;259(1):213–216. doi: 10.1016/0014-5793(89)81531-5. [DOI] [PubMed] [Google Scholar]
- Nowak L., Ascher P., Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci. 1987 Jan;7(1):101–109. doi: 10.1523/JNEUROSCI.07-01-00101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri G., Meiri H. Characterization of sodium currents in mammalian sensory neurons cultured in serum-free defined medium with and without nerve growth factor. J Membr Biol. 1990 Apr;115(1):13–29. doi: 10.1007/BF01869102. [DOI] [PubMed] [Google Scholar]
- Orozco C. B., Epstein C. J., Rapoport S. I. Voltage-activated sodium conductances in cultured normal and trisomy 16 dorsal root ganglion neurons from the fetal mouse. Brain Res. 1988 Feb 1;466(2):265–274. doi: 10.1016/0165-3806(88)90052-1. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Beckh S., Kubo H., Yahagi N., Ishida H., Kayano T., Noda M., Numa S. Functional expression of cloned cDNA encoding sodium channel III. FEBS Lett. 1988 Feb 8;228(1):195–200. doi: 10.1016/0014-5793(88)80615-x. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S., Agnew W. S. Molecular diversity of voltage-sensitive Na channels. Annu Rev Physiol. 1989;51:401–418. doi: 10.1146/annurev.ph.51.030189.002153. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S., Cooperman S. S., Tomiko S. A., Zhou J. Y., Crean S. M., Boyle M. B., Kallen R. G., Sheng Z. H., Barchi R. L., Sigworth F. J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989 Jul;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]
- White M. M., Chen L. Q., Kleinfield R., Kallen R. G., Barchi R. L. SkM2, a Na+ channel cDNA clone from denervated skeletal muscle, encodes a tetrodotoxin-insensitive Na+ channel. Mol Pharmacol. 1991 May;39(5):604–608. [PubMed] [Google Scholar]
- Yarowsky P. J., Krueger B. K. Development of saxitoxin-sensitive and insensitive sodium channels in cultured neonatal rat astrocytes. J Neurosci. 1989 Mar;9(3):1055–1061. doi: 10.1523/JNEUROSCI.09-03-01055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]