Abstract
Objective
To evaluate a novel technique designed to reduce the negative impact of motion artifacts in infant dual-energy X-ray absorptiometry (DXA) scans.
Study design
Using cross-sectional data from a large multicenter study, we developed and tested advanced methods for infant scan analysis. Newborns (n = 750) received spine and whole-body DXA scans with up to 3 attempts to acquire a motion free scan. Precision of infant DXA was estimated from visits with multiple valid scans. Accuracy of regional reflection, fusion, and omission techniques was estimated by comparing modified scans to unmodified valid scans. The effectiveness of the acquisition and analysis protocol was represented by the reduction in rate of failure to acquire valid results from infant visits.
Results
For infant whole-body DXA, arm reflection and all fusion techniques caused no significant changes to bone mineral content, bone mineral density, bone area, total mass, fat mass, lean mass, and percentage fat. Leg reflection and arm/leg dual-reflection caused significant changes to total mass, but the percentage change remained small. For infant spine DXA, fusion and omission caused no significant changes. Advanced analysis techniques reduced the failure rate of whole-body scanning from 20.8% to 9.3% and the failure rate of spine scanning from 8.9% to 2.4%.
Conclusions
Advanced analysis techniques significantly reduced the impact of motion artifacts on infant DXA scans. We suggest this protocol be used in future infant DXA research and clinical practice.
Dual-energy X-ray absorptiometry (DXA) is the gold standard for measuring bone and body composition status in adults because of its high reproducibility and precision.1 The precision of adult spine bone mineral density (BMD) and whole-body percentage fat are typically better than 1%.2,3 Evaluations of bone health are also needed to identify infants, children, and adolescents who may benefit from interventions to decrease their risk of fracture.1 A reliable method of measuring bone health during early life is necessary to optimize intervention strategies.4 In a comprehensive overview of body composition measurement modalities, Demerath and Fields5 found DXA to be ideal for use in children because of its high precision and low radiation dose. However, the precision of DXA BMD measures in children has been reported to be worse than that of adults most likely because of smaller bone sizes, lower bone densities, and movement artifacts.6 Nevertheless, the International Society for Clinical Densitometry (ISCD) considers DXA a valid method for assessing bone health in infants and recommends that spine and whole-body DXA scans be performed for pediatric bone evaluations. Several studies have found that infant bone and body composition are important markers of immediate and lifelong health.7–10 Though anthropometric measures can be used to assess infant growth in relation to World Health Organization standards, body composition varies substantially at birth.4 Body weight alone does not adequately reflect disease risk. DXA can provide a more reliable indicator of infant health in efforts to optimize intervention strategies, and DXA results have been validated to infant weight of <2 kg.11
Previous studies have shown that motion artifacts introduce unpredictable variance into DXA measurements.12,13 The official positions of the ISCD recommend that all artifacts be removed from DXA scans whenever possible.14 Various methods have been used to mitigate motion artifacts including swaddling, timing of the scan while the infant sleeps, and light sedatives.15 However, pervasive motion artifacts still represent a significant barrier to infant DXA assessment.16–19
For this study, we created guidelines on positioning and analysis for improved accuracy and precision of spine and whole-body infant DXA scanning. We evaluated the precision of infant spine and whole-body DXA scans, as well as the accuracy of regional omission, reflection, and fusion techniques. Our guidelines should improve the usability of infant DXA bone health measurements by reducing the negative influence of motion artifacts.
Methods
We performed a cross-sectional observational substudy on an existing cohort of infants who underwent spine and whole-body DXA scans. Between August 2011 and June 2014, we recruited 784 infants as part of the International Maternal Pediatric Adolescent AIDS Clinical Trials P1084s study in Africa to assess the potential impact on bone of antiretroviral drugs taken by pregnant women for prevention of HIV vertical transmission. The details to the recruitment and study design can be found elsewhere.20 To acquire motion-free DXA scans, infants received up to 3 whole-body and spine scans at birth (0–21 days of age) and at 26 weeks of age. However, in this report, we examined only the scans taken at birth. Study visits were scheduled for the morning or when the infant was napping. Infants were checked to ensure they were dry, clean, and wearing comfortable loose fitting clothing with no metal components such as buttons, pins, or snaps. Infants were allowed to use a pacifier if necessary. Infants were well fed to ensure they were as comfortable as possible during the scans. Written informed consent was obtained from all mothers in the study.
DXA Scan Acquisition
Participants from 10 study sites were scanned using 1 of 6 DXA systems; 5 were Hologic Discovery/Wi and 1 was Hologic Discovery/W (Hologic, Marlborough, Massachusetts). To minimize measurement bias because of differences in scanners and software, all DXA systems were cross-calibrated according to ISCD protocol. At no point during scanning were infants unattended. All DXA technologists received training specific to the protocol by the DXA quality assurance center at the University of California at San Francisco, California.
For spine acquisition, the infant was placed in the center of the DXA table (Figure 1; available at www.jpeds.com). The technologist felt for the top of the iliac crest, then centered the laser 2 cm below the iliac crest. Scans were taken with the fast array anteoposterior lumbar spine scan mode. The scan image was reviewed by the technologist for scan quality, including movement in the L1–L4 regions. If necessary, light restraint to the infant’s arms and/or lower body was used to keep the infant still. The restrainer’s hands were placed outside of the scan field. Images were reviewed by the technologist to ensure the spine was centrally positioned and that the top of the iliac crests, all of L5, and at least 5 vertebral bodies were included in the study (Figure 2; available at www.jpeds.com). If the image was not clearly visible because of low BMD, the scan was continued and assessed for quality after acquisition. Scans were repeated up to 2 times if movement was visible on the image or if the image quality was otherwise questionable. If the infant was noncooperative, scans were attempted again at a later time. No more than 3 attempts were made to acquire a valid infant spine scan. Properly analyzed examples of valid and invalid lumbar spine scans are shown in Figure 2.
Figure 1.
Proper positioning for spine (left) and whole-body (right) DXA scan acquisition. For spine, the infant is centered on the table top (top left) and for whole-body scanning, the infant is positioned near the top of the scanner (top right). Using this approach, the whole-body scan length and time can be minimized. During spine scanning, the infant is immobilized by an assistant holding their arms and pelvis stationary (bottom right). Light pressure could also be used to restrain the shoulders if warranted. For whole-body, swaddling is used (bottom left) to immobilize the infant.
Figure 2.
Spine analysis consisted of 4 ROIs identifying lumbar vertebrae L1, L2, L3, and L4. Identification and placement of these ROIs was done from the bottom up. L5 was identified as the vertebra between the top of the iliac crests. The bottom line of the global ROI was placed in the intervertebral space between L4 and L5. Each sequential vertebral line was placed in the subsequent intervertebral spaces, with the upper global ROI line placed between T12 and L1. Bottom, Spine scan with no motion vs a scan with obvious motion in L4.
For whole-body acquisition, the infant was swaddled in a blanket and placed near the top of the table (Figure 1). The swaddling technique is shown in Figure 3 (available at www.jpeds.com). A folded towel was used to keep the head straight. Arms and legs were placed in a relaxed position to avoid overlap with any other part of the body. Scans were taken with the infant whole-body scan mode. The caregiver remained in the examination room during the scan. Scans were repeated up to 2 additional times if movement was visible on the scan image or if the image quality was otherwise questionable. If the infant was noncooperative, scans were attempted again at a later time.
Figure 3.
Procedure for swaddling an infant for total body DXA scanning. Left, Lay the blanket or sheet out on a flat surface in the shape of a diamond. Place the infant on the blanket so that the head is toward the diamond peak. Bring the bottom of the blanket up over the legs and abdomen. Tuck the blanket under both arms. Center, Place the infant’s left arm along the side of the body and then fold the right corner of the blanket tightly across the body, tucking it behind the opposite side of the back. Right, Bring the right arm along the side of the body outside the blanket and wrap the blanket completely over and under the infant.
The maximum infant radiation dose for the baseline scan protocol was estimated using the calculations of Thomas et al21 and Blake et al.22 For the instance of an infant having to have the maximum of 3 whole-body and 3 lumbar spine DXA scans at baseline, the maximum radiation exposure to infants was less than or equal to 4.9 mrem, roughly equivalent to 5 days of radiation from natural sources at sea level. There are several international guidelines for dose to research subjects. One of the most detailed guideline is that used by the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA). ARPANSA guidelines are consistent with the recommendations from the International Commission on Radiologic Protection. ARPANSA states that annual research dose levels for a child in the age range from birth to 18 years should be constrained to not exceed 50 mrem (0.5 mSv).23 The maximum of 4.9 mrem this protocol could deliver to the infant is well below the most stringent upper constraint for the annual research radiation dose.
DXA Scan Analysis
Scan analysis was performed centrally at University of California at San Francisco using Hologic software v Apex 3.4. Spine scans were analyzed using a standard 4 vertebrae (region of interest [ROI]) analysis method (Figure 2). Any discontinuity in bone or soft tissue was coded as motion. Advanced analysis techniques were applied to regional data from standard lumbar spine ROIs. Interscan vertebral fusion replaced data for 1 or more invalid vertebrae with valid data from scans within the same visit. Intrascan omission estimated BMD by omitting invalid vertebrae within a single scan. Intrascan omission cannot be used to calculate total spine bone mineral content (BMC).
Whole-body scans were analyzed using 6 ROIs to isolate the arms, legs, head, and trunk (Figure 4; available at www.jpeds.com). Advanced analysis techniques were applied to data from scans with invalid regions. Intrascan limb reflection replaced data for an invalid limb region with data from its movement-free counterpart within the same DXA scan. Interscan fusion replaced data for invalidated head or trunk regions valid head or trunk region data from scans within the same visit. This infant reflection approach mimics the reflection with offset scanning techniques already in use for adults for large patients too wide for the scan field.24
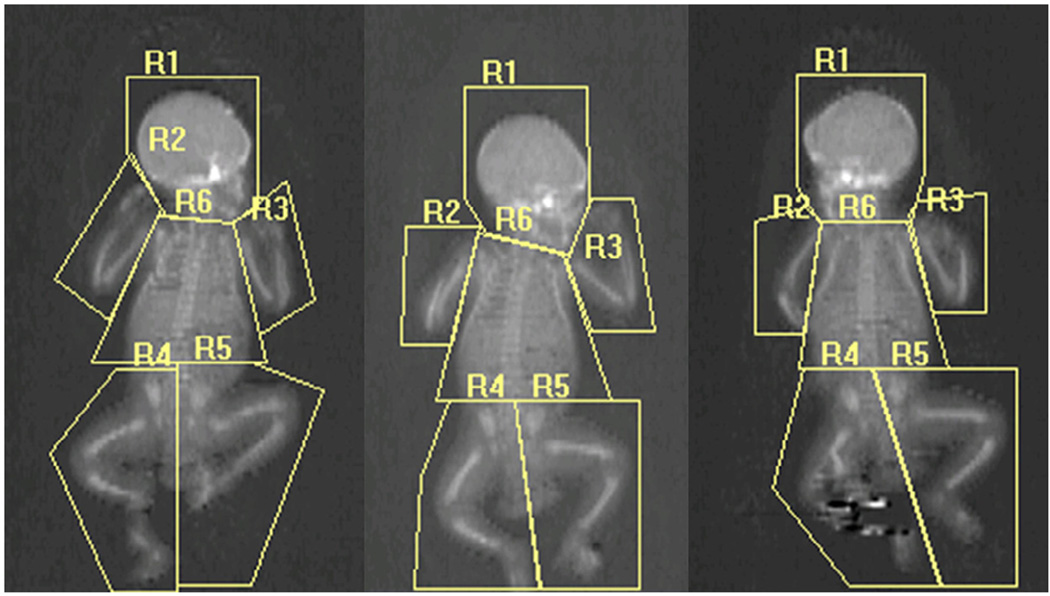
Figure 4.
Procedure for analyzing infant whole body scans. The bottom line of the head region (R1) was placed just below but not overlapping the chin, with each outside line angled up to avoid overlap with the shoulder. The right arm region (R2) and the left arm region (R3) intersected the humeral head and encompassed the entire arm and hand without overlapping the trunk or leg regions. The top lines the right leg region (R4) and the left leg region (R5) were placed just above the iliac crests. The inside lines of the leg regions were placed directly centered between each leg and foot. The trunk region (R6) encompassed all area from the bottom line of the head below the chin to the top line of the leg regions above the pelvis without overlapping any other region. Each region was individually scored as valid (no motion, no overlap with other regions, etc) or invalid. Left, Artifact-free whole-body scan showing the 6 ROI analyses. Notice that there are no overlapping regions. Center, Invalid right arm. Right, Invalid left and right arm because of overlap with trunk, movement in the right leg. Both the center and right scans had invalid BMD and % fat without using the fusion or reflection method.
Statistical Analyses
Our objective was to evaluate the accuracy and usefulness of our infant scan analysis techniques. For both whole-body and spine scans, we started with error free scans. The precision of bone and body composition measurements was estimated by root-mean-square (RMS)-coefficient of variation (%CV) and RMS-SD. Precision error and 90% CIs around the precision error were calculated according to the methods of Gluer et al.25 Paired Student t tests were used to determine technique accuracy using error-free scans with and without limb reflection, head/trunk fusion, vertebral fusion, and vertebral omission. We compared these differences to scans with motion corrected by reflection, fusion, and omission. We defined significance as an accuracy RMS-%CV greater than the point estimate of precision error RMS-%CV for any bone or body composition measurement in addition to statistically significant mean value differences with P value of <.05. The measures evaluated included BMD, BMC, bone area, fat mass, lean mass, and percentage fat. We also compared contralateral arm and leg regions with and without motion to evaluate the extent to which motion affects DXA scan measurements. Infant compliance was tested for sex differences using a χ2 proportional test. All statistical analysis was performed using SAS v 9.3 for Windows (SAS Institute Inc, Cary, North Carolina).
Results
Demographic information for all recruited infants and body composition information for infants with valid results, and no comments or codes can be found in Table I. We excluded 27 infants from analysis, 4 because of biologically unreasonable data entry errors and 23 because of baseline age greater than 4 weeks. Flowcharts showing the efficacy of reflection, fusion, and omission techniques in improving whole-body and spine scan usability can be found in Figure 5.
Table I.
Population Information
| Mean | RMS-SD | Minimum | Maximum | |
|---|---|---|---|---|
| Demographics (n = 750) | ||||
| Age (wk) | 2.1 | 0.5 | 0.0 | 3.6 |
| Scale mass (g) | 3341.6 | 544.3 | 1300.0 | 5806.0 |
| Height (cm) | 49.5 | 2.9 | 32.0 | 57.9 |
| Ponderal index (kg/cm3) | 2.8 | 0.7 | 1.5 | 10.0 |
| Whole body DXA measures (n = 521) |
||||
| BMC (g) | 68.2 | 16.0 | 28.0 | 144.0 |
| BMD (g/cm2) | 0.174 | 0.024 | 0.116 | 0.290 |
| Mass (g) | 3788.5 | 553.4 | 1899.5 | 5663.2 |
| Percentage fat (%) | 20.7 | 6.0 | 7.7 | 57.2 |
| Arm DXA measures (n = 393) | ||||
| BMC (g) | 3.4 | 0.8 | 0.9 | 5.3 |
| BMD (g/cm2) | 0.107 | 0.018 | 0.068 | 0.176 |
| Mass (g) | 185.7 | 38.8 | 64.8 | 337.9 |
| Percentage fat (%) | 25.1 | 7.2 | 8.1 | 54.0 |
| Leg DXA measures (n = 393) | ||||
| BMC (g) | 7.2 | 1.5 | 3.3 | 13.8 |
| BMD (g/cm2) | 0.137 | 0.015 | 0.099 | 0.183 |
| Mass (g) | 590.7 | 108.8 | 251.7 | 1008.2 |
| Percentage fat (%) | 24.8 | 6.6 | 8.2 | 51.9 |
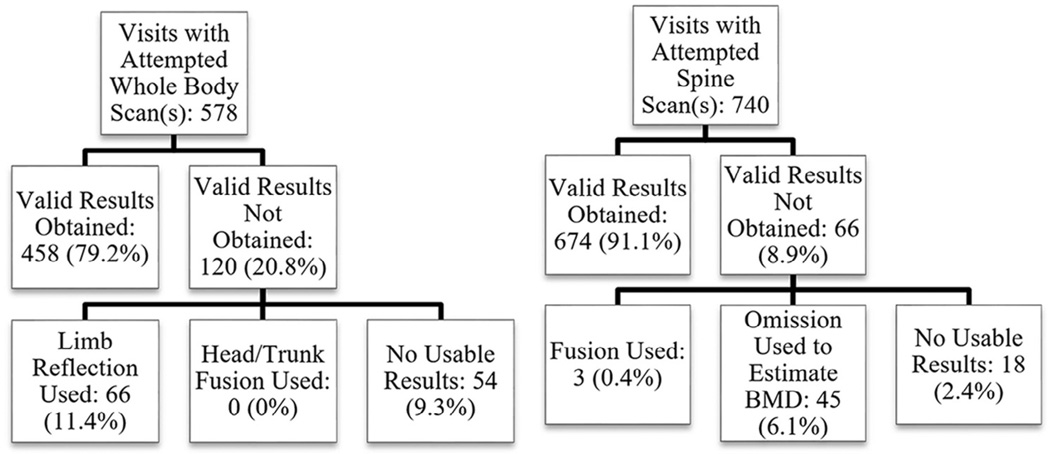
Figure 5.
Exclusion trees for whole-body and spine DXA scans. For infant whole-body, 90.7% of infants had usable visits. For spine, 97.6% of infants had usable visits.
Out of 578 unique infant whole-body scan visits with attempted scans, valid scans were obtained for 458 unique infants (79.2%). A total of 18 duplicate valid scans were obtained from 9 unique infants. Precision estimates for infant whole-body DXA based on these duplicate valid scans can be found in Table II. Sex did not significantly affect infant compliance.
Table II.
Whole-body DXA precision
| Measures | RMS-SD | RMS-%CV |
|---|---|---|
| BMC (g) | 5.1 | 5.0 (3.7, 8.2) |
| BMD (g/cm2) | 0.01 | 4.5 (3.3, 7.4) |
| Bone area (cm2) | 15.0 | 3.1 (2.3, 5.1) |
| Total mass (g) | 79.9 | 1.5 (1.1, 2.5) |
| Fat mass (g) | 132.0 | 8.4 (6.1, 13.8) |
| Lean mass (g) | 83.4 | 2.2 (1.6, 3.6) |
| Percentage fat (%) | 2.9 | 11.2 (8.2, 18.4) |
There were 9 infants with valid duplicate scans for a total of 18 scans.
To determine reflection accuracy, we eliminated all scans with any codes or comments for poor positioning or bent limbs, resulting in 199 usable scans. All usable scans were reflected left-on-right and right-on-left limb. No significant differences were found between left-on-right and right-on-left reflection. Accuracy estimates for right-on-left arm reflection, leg reflection, and dual arm/leg reflection can be found in Table III.
Table III.
Whole-body DXA analysis technique accuracy
| RMS-SD | RMS-%CV | % Change | P value | |
|---|---|---|---|---|
| Arm reflection (n = 199) | ||||
| BMC (g) | 0.36 | 0.5 | 0.07 | .46 |
| BMD (g/cm2) | 0.001 | 0.6 | 0.18 | <.01* |
| Bone area (cm2) | 2.91 | 0.7 | −0.10 | .13 |
| Total mass (g) | 52.95 | 1.3 | 1.72 | <.01* |
| Fat mass (g) | 7.60 | 0.9 | −0.06 | .30 |
| Lean mass (g) | 13.87 | 0.5 | −0.05 | .26 |
| Percentage fat (%) | 0.3 | 1.5 | −1.75 | <.01* |
| Leg reflection (n = 199) | ||||
| BMC (g) | 0.60 | 0.9 | −0.58 | <.01* |
| BMD (g/cm2) | 0.002 | 1.0 | 0.06 | .70 |
| Bone area (cm2) | 5.00 | 1.3 | −0.62 | <.01* |
| Total mass (g) | 67.45 | 1.7† | 1.84 | <.01* |
| Fat mass (g) | 10.84 | 1.3 | 0.45 | <.01* |
| Lean mass (g) | 33.53 | 1.1 | −0.04 | .63 |
| Percentage fat (%) | 0.3 | 1.3 | −1.37 | <.01* |
| Arm/leg dual-reflection (n = 199) |
||||
| BMC (g) | 0.70 | 1.0 | −0.51 | <.01* |
| BMD (g/cm2) | 0.002 | 1.2 | 0.24 | .09 |
| Bone area (cm2) | 5.94 | 1.5 | −0.72 | <.01* |
| Total mass (g) | 68.85 | 1.8† | 1.78 | <.01* |
| Fat mass (g) | 13.81 | 1.6 | 0.39 | .01* |
| Lean mass (g) | 35.13 | 1.2 | −0.09 | .38 |
| Percentage fat (%) | 0.3 | 1.5 | −1.37 | <.01* |
| Head fusion (n = 18) | ||||
| BMC (g) | 19.82 | 18.7 | −5.37 | .31 |
| BMD (g/cm2) | 0.019 | 9.4 | −2.81 | .43 |
| Bone area (cm2) | 30.05 | 6.1 | −3.09 | .18 |
| Total mass (g) | 219.35 | 3.9 | 0.72 | .89 |
| Fat mass (g) | 137.83 | 8.1 | −1.06 | .25 |
| Lean mass (g) | 136.54 | 3.4 | −0.63 | .48 |
| Percentage fat (%) | 1.5 | 5.5 | −1.94 | .21 |
| Trunk fusion (n = 18) | ||||
| BMC (g) | 5.82 | 5.1 | −0.95 | .48 |
| BMD (g/cm2) | 0.003 | 1.3 | −5.37 | .58 |
| Bone area (cm2) | 19.24 | 3.8 | −0.56 | .53 |
| Total mass (g) | 393.40 | 6.8 | 0.36 | .77 |
| Fat mass (g) | 96.22 | 5.4 | −3.26 | .24 |
| Lean mass (g) | 345.72 | 8.6 | −1.07 | .52 |
| Percentage fat (%) | 1.197 | 4.3 | −3.42 | .13 |
P < .05.
RMS-%CV greater than whole body DXA precision.
For arm reflection, we found differences between reflected and nonreflected scans of 0.18%, 1.72%, and −1.75% for BMD, total mass, and percentage fat, respectively. However, the accuracy RMS-%CVs for all measurements were below the whole-body precision RMS-%CVs, meaning the accuracy of arm reflection was better than the precision of infant whole-body DXA scans. As such, arm reflection did not meet our a priori criteria for significant change.
For leg reflection, we found differences of −0.58%, 1.81%, −1.37%, 0.45%, and −0.62% for BMC, total mass, percentage fat, fat mass, and bone area, respectively. The leg-reflected total mass RMS-%CV of 1.7% was larger than the duplicate valid scan total mass RMS-%CV of 1.5%. Because the total mass accuracy of scans with leg reflection was worse than the precision of infant whole-body DXA, it failed our a priori criteria for nonsignificance. Leg reflection may significantly impact infant whole-body DXA total mass results. However, it is worth noting that all leg reflection accuracy RMS-%CV’s were well within the 90% CI around the precision error RMS-%CV as shown in Tables II and III.
For dual arm/leg reflection we found differences of −0.51%, −0.72%, 1.78%, 0.39%, and −1.37% for BMC, bone area, total mass, fat mass, and percentage fat, respectively. Similar to leg reflection, the arm/leg dual-reflected total mass RMS-%CV of 1.8% was larger than the duplicate valid scan total mass RMS-%CV of 1.5%. Because the total mass accuracy of scans with arm/leg dual-reflection was lower than the precision of infant whole-body DXA, arm/leg dual-reflection may significantly impact infant whole-body DXA total mass results. As with leg reflection, all dual arm/leg reflection accuracy RMS-%CVs were well within the 90% CI around the precision error RMS-%CV.
We analyzed head fusion and trunk fusion using duplicate valid whole-body scans. Accuracy estimates for fusion can be found in Table III. We found no significant differences between fused and nonfused scans using either head fusion or trunk fusion. We did not test dual-head/trunk fusion within the same scan.
A sample of 417 whole body scans with no arm motion, 467 scans with no leg motion, 42 scans with motion in one arm, and 57 scans with motion in one leg was used to compare contralateral regional measurements of BMC, BMD, percentage fat, and mass. Motion artifacts increased the SD of the difference between contralateral regions by an average of 42% for BMD, 80% for BMC, 38% for percentage fat, and 98% for mass (Table IV; available at www.jpeds.com).
Table IV.
Contralateral DXA regional comparison with and without motion on 1 side
| RMS-SD | |
|---|---|
| Arm left vs right difference with motion (n = 42) |
|
| BMD (%) | 30.7 |
| BMC (%) | 45.9 |
| Mass (%) | 20.0 |
| Percentage fat (%) | 10.7 |
| Arm left vs right difference with no motion (n = 417) |
|
| BMD (%) | 13.2 |
| BMC (%) | 15.5 |
| Mass (%) | 14.2 |
| Percentage fat (%) | 10.6 |
| Leg left vs right difference with motion (n = 57) |
|
| BMD (%) | 14.6 |
| BMC (%) | 28.0 |
| Mass (%) | 26.6 |
| Percentage fat (%) | 7.2 |
| Leg left vs right difference no motion (n = 467) |
|
| BMD (%) | 10.7 |
| BMC (%) | 9.3 |
| Mass (%) | 9.8 |
| Percentage fat (%) | 5.4 |
Out of 740 unique infant spine scan visits with attempted scans, valid scans were obtained for 674 unique infants (91.1%; Figure 5). We analyzed vertebral fusion using 40 duplicate valid spine scans representing 20 unique infants. Out of all 20 possible combinations of BMC and BMD measures for single and double vertebra fusion, the lowest P value was .072. We found no significant differences between fused and nonfused scans using either single or double vertebral fusion.
In analysis of vertebral omission accuracy, we eliminated all scans with any codes or comments, resulting in 46 usable scans. BMC was not estimated using omission techniques. Out of all 10 possible combinations of BMD measures for single and double vertebra omission, the lowest P value was .122. We found no significant differences in BMD between altered and nonaltered scans using either single or double vertebral omission.
For whole-body scans, limb reflection was used to generate data for 11.4% of all attempted scan visits. Head/trunk fusion was not used on any scan. Overall, reflection reduced the whole-body scan failure rate from 20.8% to 9.3%.
For spine scans, vertebra fusion was used to obtain BMC and BMD data for 0.4% of all attempted scan visits. Vertebra omission was used to obtain BMD on 6.1% of all attempted scan visits. Overall, fusion and omission reduced the spine scan BMC failure rate from 8.9% to 8.5% and the BMD failure rate from 8.9% to 2.4%.
Discussion
In this study, we proposed new guidelines for scan acquisition and postscan analysis designed to minimize the negative impact of pervasive motion artifacts. We tested the accuracy and usefulness of reflection, omission, and fusion methods for infant whole-body and spine DXA scans. For whole-body scans, neither arm reflection nor head/trunk fusion significantly impacted the precision or accuracy of the scan results. However, leg reflection and arm/leg dual-reflection resulted in small but significant changes of 1.84% and 1.78% in total mass, respectively. For spine scans, both fusion and omission produced accurate results. However, fusion should be used before omission if possible to salvage BMC data as well as BMD data. In addition, we observed large increases in measurement variance caused by motion artifacts in arm and leg regions when compared with motion-free scans. Overall, using reflection, fusion, and omission reduced the failure rate of infant whole body and spine DXA visits by more than one-half. Thus, advanced analysis techniques provided a means to salvage whole-body scans that would normally be rejected, with only a small impact on accuracy.
Using the methods outlined in this article, we were able to acquire usable results in 90.7% of whole-body scan visits and 97.6% (91.4% BMC) of spine scan visits. As a comparison, Kalkwarf et al16 acquired DXA infant spine scans on 369 ranging in age from 1 to 36 months. In this age group of less than 6 months, 73% of the infants had acceptable scans without movement even though a similar restraining method was used. However, only 1 scan attempt was made. We attribute our higher percentage of usable spine scans to allowing up to 3 attempts at achieving a motion-free scan.
A strength of our protocol and its validation is that the infant scans were acquired on multiple DXA systems by multiple DXA technologists, cross-calibrated according to standard ISCD methods. Thus, the protocol demonstrates robustness even with these differences in infant positioning and handling. Based on this robustness, clinical DXA sites should have a similar success rate. No scanner currently has software that automatically performs the functions described in this manuscript. Clinicians and researchers can perform reflection, fusion, and omission using data from standard ROIs (GE systems, Chicago, Illinois) or manually defined ROIs (Hologic systems). Thus, the methods described here are universally applicable. This study had some limitations. The DXA scanning protocol called for scanning to stop after a single movement-free scan was acquired. This limited the number of duplicate valid scans, which are required to analyze fusion techniques and scan precision. Because of the limited number of duplicate valid scans, our statistical power varied between precision and accuracy tests of reflection, fusion, and omission. Because we were blinded to exposure, we could not evaluate differences between treatment and control groups for Tenofovir exposure. However, we would not expect treatment to make significant differences to isolation and correction of movement artifacts.
We conclude that this protocol increased the number of usable infant DXA scans. This protocol should improve the power of clinical studies involving infant bone and body composition measures.
Acknowledgments
We would like to thank the DXA technicians: Benjamin Bika, Owen Daire, Rhinesha Dayanand, Riana Eagar, Sam Kampondeni, Terry Mazuba, Cynthia Mukwasi-Kahari, Irene Nalweyiso, Thabile Sibiya, Eliza Tsoeu, and Admire Zanga.
Funded by U.S. Department of Health and Human Services (UM1AI068616), National Institutes of Health (UM1AI068632), and National Institute of Allergy and Infectious Diseases (UM1AI106716).
Glossary
- ARPANSA
Australian Radiation Protection and Nuclear Safety Agency
- BMC
Bone mineral content
- BMD
Bone mineral density
- DXA
Dual-energy X-ray absorptiometry
- ISCD
International Society for Clinical Densitometry
- ROI
Region of interest
- RMS
Root-mean-square
- %CV
Coefficient of variation
Footnotes
The authors declare no conflicts of interest.
References
- 1.Gordon CM, Leonard MB, Zemel BS. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17:219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Fan B, Lewiecki EM, Sherman M, Lu Y, Miller PD, Genant HK, et al. Improved precision with Hologic Apex software. Osteoporos Int. 2008;19:1597–1602. doi: 10.1007/s00198-008-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd JA, Fan B, Lu Y, Lewiecki EM, Miller P, Genant HK. Comparison of BMD precision for Prodigy and Delphi spine and femur scans. Osteoporos Int. 2006;17:1303–1308. doi: 10.1007/s00198-006-0127-9. [DOI] [PubMed] [Google Scholar]
- 4.International Atomic Energy Agency. IAEA Human Health Series No. 22. Vienna: IAEA; 2013. Body composition assessment from birth to two years of age. [Google Scholar]
- 5.Demerath EW, Fields DA. Body composition assessment in the infant. Am J Hum Biol. 2014;26:291–304. doi: 10.1002/ajhb.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepherd JA, Wang L, Fan B, Gilsanz V, Kalkwarf HJ, Lappe J, et al. Optimal monitoring time interval between DXA measures in children. J Bone Miner Res. 2011;26:2745–2752. doi: 10.1002/jbmr.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt MD, Dwyer T, Magnussen CG, Venn AJ. Predictive associations between alternative measures of childhood adiposity and adult cardiometabolic health. Int J Obes. 2010;35:38–45. doi: 10.1038/ijo.2010.205. [DOI] [PubMed] [Google Scholar]
- 8.Elks CE, Loos RJF, Sharp SJ, Langenberg C, Ring SM, Timpson NJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 2010;7:e1000284. doi: 10.1371/journal.pmed.1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity (Silver Spring) 2012;20:165–171. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis KJ. Body composition in infancy: impact on health later in life. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:213–220. doi: 10.1159/000281168. discussion 21-4. [DOI] [PubMed] [Google Scholar]
- 11.Rigo J, Nyamugabo K, Picaud JC, Gerard P, Pieltain C, De Curtis M. Reference values of body composition obtained by dual energy X-ray absorptiometry in pretermand term neonates. J Pediatr Gastroenterol Nutr. 1998;27:184–190. doi: 10.1097/00005176-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Gilsanz V. Bone density in children: a review of the available techniques and indications. Eur J Radiol. 1998;26:177–182. doi: 10.1016/s0720-048x(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 13.Cawkwell GD. Movement artifact and dual X-ray absorptiometry. J Clin Densitom. 1998;1:141–147. [Google Scholar]
- 14.Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The official positions of the International Society for Clinical Densitometry: acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom. 2013;16:520–536. doi: 10.1016/j.jocd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree NJ, Kent K, Zemel BS. Bone densitometry in growing patients. Totowa (NJ): Humana Press; 2007. Acquisition of DXA in Children and Adolescents; pp. 73–91. [Google Scholar]
- 16.Kalkwarf HJ, Zemel BS, Yolton K, Heubi JE. Bone mineral content and density of the lumbar spine of infants and toddlers: influence of age, sex, race, growth, and human milk feeding. J Bone Miner Res. 2013;28:206–212. doi: 10.1002/jbmr.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalkwarf HJ, Abrams SA, DiMeglio LA, Koo WW, Specker BL, Weiler H. Bone densitometry in infants and young children: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:243–257. doi: 10.1016/j.jocd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Koo WW, Massom LR, Walters J. Validation of accuracy and precision of dual energy X-ray absorptiometry for infants. J Bone Miner Res. 1995;10:1111–1115. doi: 10.1002/jbmr.5650100716. [DOI] [PubMed] [Google Scholar]
- 19.Koo WW, Walters J, Bush AJ, Chesney RW, Carlson SE. Dual-energy X-ray absorptiometry studies of bone mineral status in newborn infants. J Bone Miner Res. 1996;11:997–1002. doi: 10.1002/jbmr.5650110717. [DOI] [PubMed] [Google Scholar]
- 20.IMPAACT Study P1084s. Maternal and infant monitoring for evidence of toxicity related to tenofovir exposure: the bone and kidney health substudy of the IMPAACT 1077 PROMISE Protocol. 2016 IMPAACT P1084s (DAIDS Document ID 10790); http://impaactnetwork.org/DocFiles/P1084s/P1084s_V2_11Oct12_CM1.pdf. [Google Scholar]
- 21.Thomas S, Kalkwarf H, Buckley D, Heubi J. Effective dose of dual-energy X-ray absorptiomety scans in children as a function of age. J Clin Densitom. 2005;8:415–422. doi: 10.1385/jcd:8:4:415. [DOI] [PubMed] [Google Scholar]
- 22.Blake GM, Naeem M, Boutros M. Comparison of effective dose to children and adults from dual X-ray absorptiometry examinations. Bone. 2006;38:935–942. doi: 10.1016/j.bone.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 23.ARPANSA. Code of Practice for the Exposure of Humans to Ionizing Radiation for Research Purposes, Radiation Protection Series No. 8. 2005:1–28. http://www.arpansa.gov.au/publications/codes/rps8.cfm. [Google Scholar]
- 24.Baim S, Leonard MB, Bianchi M-L, Hans DB, Kalkwarf HJ, Langman CB, et al. Official Positions of the International Society for Clinical Desitometry and executive summary of the 2007 ISCD Position Develpment Conference. J Clin Densitom. 2008;11:75–91. doi: 10.1016/j.jocd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]