Figure 1.
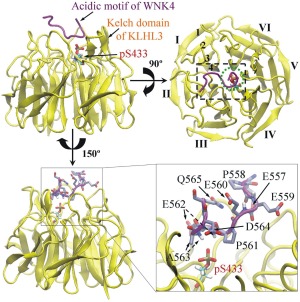
The initial positions of modeled phosphorylated S433 (pS433) in the Kelch domain of KLHL3 (residues 300–585, in yellow) and residues in the acidic motif (AM, residues 557 − 565, in purple) of WNK4. In the top view (upper right), the six Kelch repeats in the Kelch domain of KLHL3 are labeled as I–VI, and four antiparallel β‐strands in repeat I are labeled as 1–4. The black dashed rectangle represents the AM‐binding site on Kelch domain of KLHL3, and the green dot circle represents the location of pS433 in the top view. For clarity, residues of the AM in WNK4 and pS433 of KLHL3 are enlarged (lower right). The pS433 residue is located in the pocket formed by the side chain of P561, main chain of E562 and both main and side chains of A563 in the AM of WNK4. For simplicity, the hydrogen atoms in the side chain of AM are not shown.
