Figure 4.
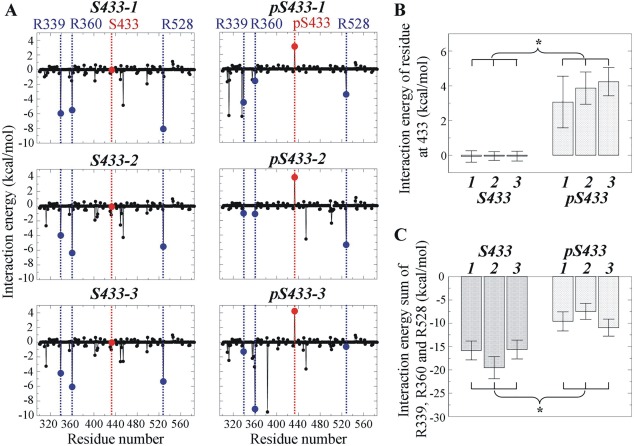
Interaction energy for residues of KLHL3 involved in the formation of intermolecular hydrogen bonds between acidic motif and Kelch domain. (A) Interaction energy for residues R339, R360, and R528 in Kelch domain containing S433 or pS433. Blue dash lines indicate the positions of these key interaction residues and red dash lines indicate the positions of S433 or pS433. (B) Interaction energy at S433 rose significantly after phosphorylation. (C) The sum of interaction energy for R339, R360, and R528 decreased significantly upon phosphorylation at S433. For (B and C), three simulations (labeled as 1, 2, and 3) were performed in the presence and in the absence of phosphorylation at S433, respectively. * indicates that the difference is statistically significant based on the mean and standard deviation of the analyzed variable (Student's t test, P < 0.05).
