Figure 1.
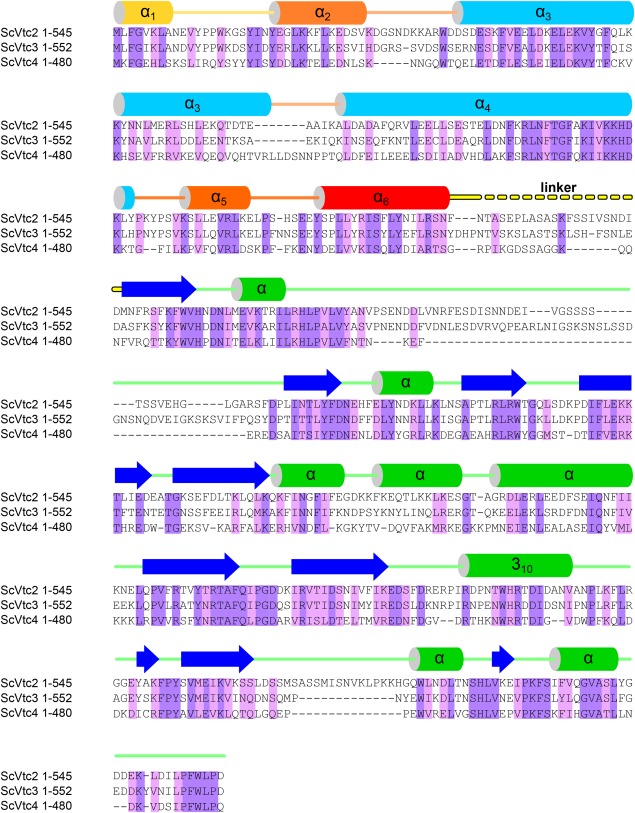
The VTC complex harbors N‐terminal SPX domains. Structure based sequence alignment of VTC subunits ScVtc2 (S. cerevisiae Vtc2; UniProt Acc. P43585), ScVtc3 (S. cerevisiae Vtc3; Q02725) and ScVtc4 (S. cerevisiae Vtc4; P47075) and including a secondary structure assignment calculated with the program DSSP48 and based on PDB entry 5IIG.14 Invariant and conserved residues are highlighted in dark‐ and light‐purple, respectively. SPXScVtc 2 shows 62% and 32% sequence identity with SPXScVtc 3 and SPXScVtc 4, respectively. The two long core helices of the SPX domain are colored in light blue, surrounding helices are colored from yellow to red. The C‐terminal catalytic TTM domain (α‐helices in green, β‐strands in blue) is connected to the SPX domain via a variable linker (in yellow).
