Figure 3.
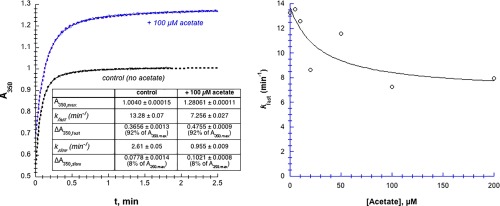
Acetate moderately inhibits Hg2+‐induced aggregation of chymotrypsin. [chymotrypsin] = 20 µM, 50 mM MOPS at pH 7.23, [Hg2+] = 1.025 mM. (A) A350 transients are fit to double‐first order kinetics, that is, a fast first order phase followed by a slower first order phase (Eq. (5), dashed lines). (B) Data were fit to Eq. (3), but modified to decline to a non‐zero value (k fast,final) at infinite [acetate]. Parameters for the fitted curve are: k fast,0 = 14 ± 4 µM min−1, k fast,final = 6.9 ± 2.5 µM min−1, and IC50 = 30 ± 40 µM, R 2 = 0.71. Uncertainties in the first order fitted values of k are smaller than the point symbols.
