Figure 4.
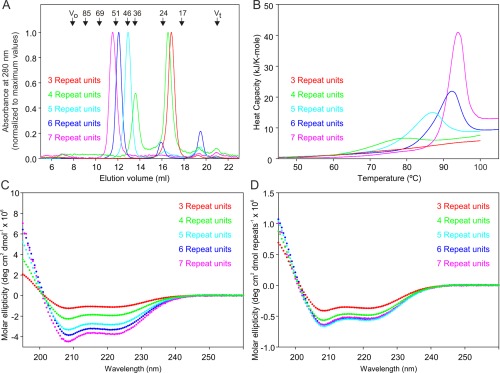
Analysis of the stability and conformation of uniform neck domains containing different numbers of repeat units with leucine at position 6. (A) Gel filtration analysis showing decreasing molecular weight of the tetramer for the shorter forms and dissociation of the two smallest versions. (B) Differential scanning calorimetry reveals reduced denaturation temperatures for the shorter forms of the neck. Protein concentrations were 210, 210, 220, 189, and 94 μM for the polypeptides with 3, 4, 5, 6, and 7 repeat units, respectively. (C) Circular dichroism spectra expressed per mole of polypeptide. Protein concentrations were 21, 16, 6.2, 6.7, and 10 μM for the polypeptides with 3, 4, 5, 6, and 7 repeat units, respectively. (D) Circular dichroism spectra normalized to number of repeat units to demonstrate that all spectra have the same shape and only the shortest forms differ in the intensity of the signals.
