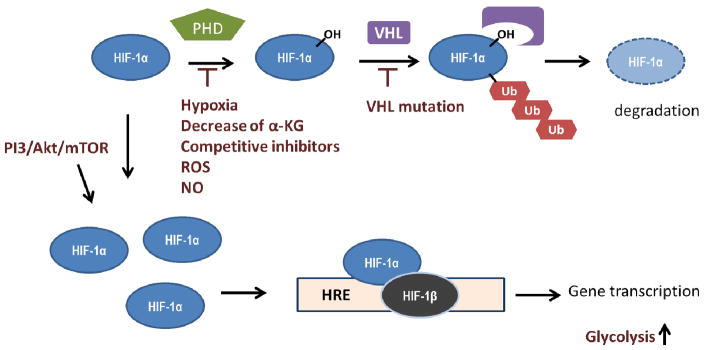
Figure 1.1. Mechanisms of HIF-1α activation in cancers.
In normoxia, hypoxia-inducible factor (HIF)-1α is hydroxylated by prolyl hydroxylase domain proteins (PHD), which uses oxygen and α-KG as substrates. Hydroxylated HIF-1α is recognized by von Hippel-Lindau (VHL), which facilitates HIF-1α ubiquitination, and such modification subjects HIF-1α to proteasomal degradation. Upregulation of HIF-1 signaling has been observed in many cancers caused by aberrant activation of PI3K/Akt/mTOR pathway leading to an increase of HIF-1α translation. The inhibition of PHD by decrease of α-KG, increase of competitive inhibitors, and inactivation of enzyme activity mediated by reactive oxidative species (ROS) or NO have been observed. In addition, loss-of-function mutation of VHL also contributes to HIF-1α stabilization. HIF-1α associates with HIF-1β and binds to the hypoxia response element (HRE) of promoters resulting in transcription of target genes, including those involved in glycolysis regulation. Herein, HIF-1α stabilization contributes to tumor glycolytic phenotype.