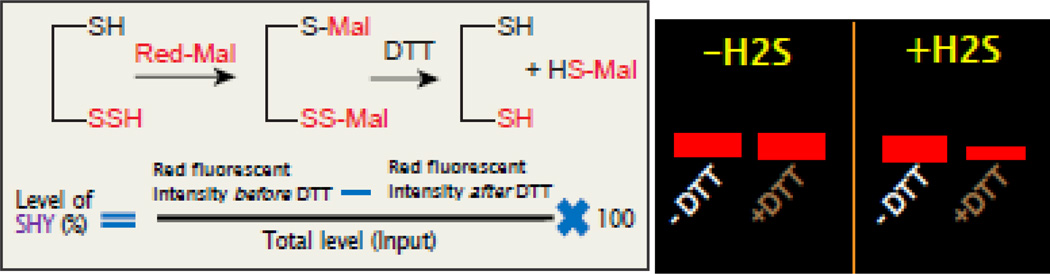
Figure 2.
The principle of red maleimide assay to detect sulfhydration of proteins. In this method, red malimide interacts with both –SH and -SSH groups and forms –S-Mal and –S-S-Mal respectively. Upon treatment with DTT, the –S-Mal will remain unaltered, however, –S-S-Mal will be cleaved off to form –SH and –S-Mal as the products. Thus the sulfhydrated protein will lose the signal of red fluorescence of red maleimide. A cartoon image (right side) of a gel showing that the red intensity of a target protein remains unaltered in the presence or absence of DTT in samples untreated with H2S. On the other hand, the red fluorescent intensity of protein will be lost after DTT treatment in samples treated with H2S. The residual red fluorescent intensity before and after DTT tretament divided by total protein will provide the percentage of sulfhydration of protein.