Abstract
Malignant mesothelioma (MM) incidence is increasing drastically worldwide as an occupational disease resulting from asbestos exposure. However, no curative treatment for MM of advanced stage is available. Thus, new therapeutic approaches for MM are required. Because malignant pleural mesothelioma (MPM) cells spread along the pleural surface in most patients, MPM can be targeted using intrapleural therapeutic approaches. In this study, we investigated the effectiveness of the intrapleural instillation of a replication‐competent adenovirus as an oncolytic agent against MPM. We constructed a vascular endothelial growth factor promoter‐based conditionally replicative adenovirus (VEGF‐CRAd) that replicates exclusively in VEGF‐expressing cells. All of the MM cell lines that we tested expressed VEGF mRNA, and VEGF‐CRAd selectively replicated in these MM cells and exerted a direct concentration‐dependent oncolytic effect in vitro. Furthermore, our in vivo studies showed that pre‐infection of MM cells with VEGF‐CRAd potently suppressed MPM tumor formation in nude mice, and that intrapleural instillation of VEGF‐CRAd prolonged the survival time of tumor‐bearing mice. Our results indicate that VEGF‐CRAd exerts an oncolytic effect on MM cells and that intrapleural instillation of VEGF‐CRAd is safe and might represent a promising therapeutic strategy for MPM.
Keywords: Conditionally replicative adenovirus, gene therapy, intrathoracic nude‐mouse model, malignant mesothelioma, vascular endothelial growth factor
Malignant mesothelioma (MM), an asbestos‐related neoplasm of the mesothelium, is a locally aggressive tumor. The incidence of MM has been increasing markedly worldwide, and an analysis of mesothelioma mortality recorded in the World Health Organization (WHO) mortality database between 1994 and 2008 yielded an age‐adjusted mortality rate of 4.9 per million.1 Although MM incidence is expected to peak or remain at a high level by the years 2010–2040,2, 3 conventional therapies for MM comprising chemotherapy, surgery, and radiotherapy offer little survival benefit,4 and only the use of these therapies in combination has shown some benefit in very highly selected groups of patients.5 Therefore, the development of innovative therapies for MM is urgently needed.
Angiogenesis has been widely reported to be essential for tumor growth, invasion, and metastasis,6, 7 and tumor cells are recognized to produce numerous angiogenic cytokines and growth factors that play key roles in these steps. We previously reported that interleukin‐6 stimulation increased the expression of vascular endothelial growth factor (VEGF) in MM cell lines via the signal transducer and activator of transcription 3 (STAT3) pathway.8 Previous studies have reported the expression of VEGF mRNA, protein, and receptor in MM and have suggested the existence of VEGF‐dependent autocrine growth mechanisms in MM.9, 10 Moreover, VEGF expression was reported to be considerably higher in MM than in normal mesothelial cells,11 and serum VEGF level was found to a negative prognostic factor in MM patients;12 These findings indicate that VEGF plays a pivotal role in MM growth and is a potential target molecule for new MM therapies. Notably, in in vitro studies, the viability of mesothelioma cells was diminished following anti‐VEGF treatments performed using antisense VEGF oligonucleotides, VEGF‐neutralizing antibodies, VEGF‐diphtheria toxin protein, and antibodies targeting VEGF receptor.13 Furthermore, anti‐VEGF strategies have shown promise in several VEGF‐expressing malignancies including MM, and these strategies have been verified in clinical trials.14, 15
In the treatment of MM, an area of research that is particularly active and holds considerable promise is gene therapy. MM is a highly suitable tumor for direct gene delivery because of its natural characteristics: (i) MM mortality primarily results from local spreading, with metastasis occurring late in tumor progression; (ii) MM is unresponsive to conventional therapies; (iii) the expected inflammatory response in the treatment is likely to be better tolerated than that in the brain or lungs; and (iv) the pleural space where malignant pleural mesothelioma (MPM) occurs is readily accessible for both virus delivery and specimen acquisition for analysis.16 Treatment strategies involving the use of non‐replicative viruses, such as Ad‐HSV‐TK, have been studied and have already been tested clinically; however, non‐replicative viruses transfer genes only into the superficial layers of tumor cells, and virus penetration into the tumor is likely to be limited.17 In this regard, a conditionally replicative adenovirus (CRAd) might exhibit superior intratumoral spread and penetration because of the replication ability of the virus, and exert a stronger oncolytic effect on intrapleural tumors. In clinical trials, certain CRAds have shown promise in the treatment of ovarian cancer18 and bladder cancer,19 and in the case of MM, a midkine‐promoter‐regulated oncolytic Ad showed viral replication and oncolytic effects both in vitro and in vivo.20
Based on the aforementioned findings, we used the VEGF promoter and generated a VEGF promoter‐based CRAd, VEGF‐CRAd, whose promoter is activated in, and which can replicate in, VEGF‐positive cells. Furthermore, we investigated the oncolytic effects of VEGF‐CRAd on MM in vitro and in vivo.
Materials and Methods
Cell culture
We obtained five mesothelioma cell lines, NCI‐H28, NCI‐H226, NCI‐H2052, NCI‐H2452, and MSTO‐211H, and HEK293, an Ad‐transformed human embryonic kidney cell line, from American Type Culture Collection (Manassas, VA, USA). The mesothelioma cells were cultured in RPMI1640 (GIBCO, Grand Island, NY, USA) and the HEK293 cells were cultured in DMEM/F12 (GIBCO) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin‐streptomycin, and maintained at 37°C and 5% CO2.
Adenoviral vectors
Recombinant adenoviral vectors expressing firefly luciferase were constructed through homologous recombination in Escherichia coli by using the AdEasy system.21 Ad5VEGFE1, Ad5CMVE1 (serotype 5 adenovirus vector expressing the E1 gene driven by the VEGF promoter or CMV promoter, respectively), Ad5/3VEGFE1 and Ad5/3CMVE1 (chimeric serotype 5 adenoviral vector with serotype 3 knob expressing the E1 gene driven by the VEGF promoter or CMV promoter, respectively) and Ad5VEGFLuc (serotype 5 adenovirus vector expressing firefly luciferase gene driven by VEGF promoter) were generated as reported previously.22 A schematic representation of the recombinant adenovirus construction is shown in Figure 1.
Figure 1.
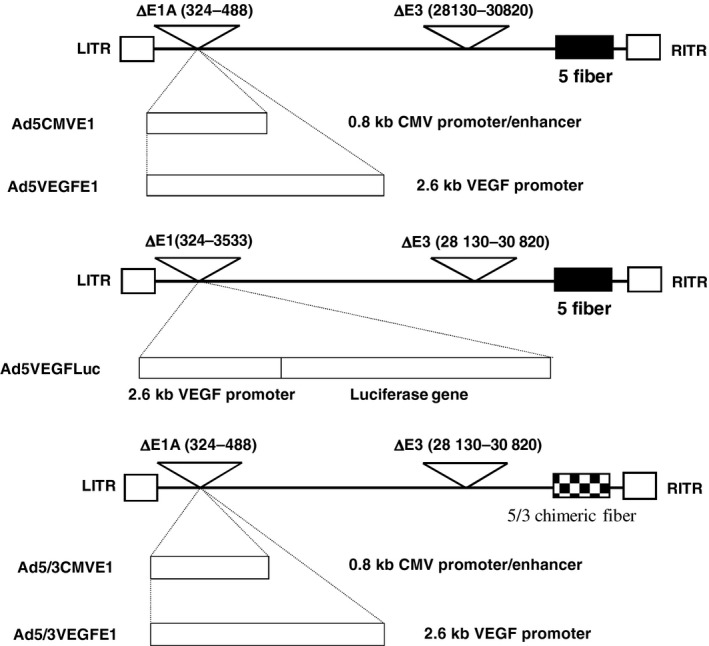
Schematic diagram of vector construction. These vectors are constructed from an E3 region‐deleted Ad5 backbone and do not contain the Ad E1A promoter region (from nucleotide 324 to 488 of the Ad genome). Deletion of the E3 region was necessary because the 2.6 kb VEGF promoter we chose was too long to insert into the Ad genome without deletion of the adenoviral E3 region. Ad5CMVE1 and Ad5VEGFE1 differ in the promoter driving E1A expression. We also constructed fiber modified Ad5/3VEGFE1 and Ad5/3CMVE1.
The viruses were propagated in the Ad‐packaging cell line HEK293, purified using double CsCl density‐gradient centrifugation, and then dialyzed against phosphate‐buffered saline containing 10% glycerol. The viral particle (VP) concentration was determined spectrophotometrically, using a conversion factor of 1.1 × 1012 VPs per absorbance unit at 260 nm,23 and standard plaque assays on HEK293 cells were performed to quantify the infectious particles.24
Analysis of VEGF RNA expression
Vascular endothelial growth factor RNA expression in each cell line was analyzed using reverse transcription and PCR (RT‐PCR) as described previously.25 Total cellular RNA was extracted from 1 × 107 cells by using the RNeasy kit (Qiagen, Valencia, CA, USA) and analyzed for VEGF and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) RNAs by using the GeneAmp RNA PCR core kit (Applied Biosystems, Grand Island, NY, USA) per the manufacturer's instructions. Briefly, 500 ng of total RNA was reverse‐transcribed with random hexamers and murine leukemia virus reverse transcriptase (50°C, 30 min) and PCR‐amplified with the primer pairs listed below (with each primer included at 50 nM) by using the following cycling program: an initial step of 95°C for 15 min, followed by 27 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min, and a final step of 72°C for 10 min. For the analyses, we used these primers: VEGF sense (5ʹ‐GAAGTGGTGAAGTTCATGGATGTC‐3ʹ) and VEGF antisense (5ʹ‐CGATCGTTCTGTATCAGTCTTTCC‐3ʹ); and GAPDH sense (5ʹ‐CCTTCATTGACCTCAACTA‐3ʹ) and GAPDH antisense (5ʹ‐GGAAGGCCATGCCAGTGAGC‐3ʹ).
In vitro analysis of VEGF promoter activation
The activity of the VEGF promoter in an adenoviral context was analyzed by infecting cells with luciferase expression vectors as reported previously.26 Briefly, cells were seeded in 12‐well plates in triplicate at a density of 1 × 105 cells/well, and on the following day, they were infected with Ad5VEGFLuc or Ad5CMVLuc at an MOI of 10 pfu/cell in DMEM containing 2% fetal calf serum for 3 h and then maintained in complete medium. The cells were also infected with Ad5/3CMVLuc to assess the difference in infectivity between Ad5 and Ad5/3 chimeric vectors. The infected cells were harvested and treated with lysis buffer (cat #E153A; Promega, WI, USA) after culturing for 2 days, and a luciferase assay (Luciferase Assay System; Promega) was used to measure the luciferase activity of the Ad‐infected cells. The measured luciferase activity was normalized relative to the protein concentration in the cell lysates (Bio‐Rad DC Protein Assay kit; Bio‐Rad, Hercules, CA, USA).
In vitro analysis of viral replication
Replication of Ads in mesothelioma cells was assessed as follows. NCI‐H226 cells were plated in six‐well plates in triplicate at a density of 3 × 105 cells/well, and after overnight culture, they were infected with each Ad construct at an MOI of 10 pfu/cell in RPMI 1640 containing 2% FBS for 3 h. Next, the infected cells were harvested and divided into three portions, re‐plated into three wells of 24‐well plates, and maintained in complete medium. The cells from a single well were harvested on Days 0 (control), 3, and 6, and the infectious particles were quantified using standard plaque assays.
In vitro cytotoxicity assay
To measure virus‐mediated cytotoxicity, 5 × 103 mesothelioma cells (NCI‐H2052, NCI‐H2452, NCI‐H226, and NCI‐H28) were seeded per well in 96‐well plates in triplicate, and after overnight culture, they were infected with each Ad construct at various MOI for 3 h. The infection medium was then replaced with RPMI1640 containing 10% FBS, and the viable cells were evaluated once every 3 days by using the MTS assay (CellTiter 96 Aqueous Non‐Radioactive Cell Proliferation Assay; Promega); the MTS color development was quantified as the optical density at 490 nm by using an EL 800 Universal Microplate Reader (Biotec Instruments Inc., Winooski, VT, USA).
To visualize the cytotoxic effect, crystal violet staining was also performed. We seeded 2 × 105 cells per well in 12‐well plates and infected the cells with each Ad construct at various MOI for 3 h, and then replaced the infection medium with growth medium on the next day. When cell lysis was observed, the cells were fixed and stained with 1% crystal violet in 70% ethanol for 45 min, and then washed with tap water to remove excess color. The plates were dried, and images were captured using a Kodak DC260 digital camera (Eastman Kodak, Rochester, NY, USA). All experiments were performed in duplicate wells.
In vivo studies: tumor formation in nude mice
All animals were used in accordance with protocols approved by the animal care committees of Kyushu University, and experiments were conducted according to both the Guidelines for Animal Experiments of Kyushu University and the Law (No. 105) and Notification (No. 6) of the Japanese government. As the animal model for studying the time course of tumor formation in vivo, we used 5‐week‐old female nude mice. Briefly, NCI‐H226 cells (1 × 107) were directly injected into the left pleural space of the nude mice (Day 1), and the mice were sacrificed every 2 weeks and the bilateral lungs, pleural walls, heart, and diaphragm were resected to check for tumor formation microscopically.
In vivo studies: suppression of tumor formation and tumor growth in nude mice
To evaluate the inhibition of tumor formation by the replication‐competent Ad, intact NCI‐H226 cells (9 × 106) mixed with NCI‐H226 cells (1 × 106) infected with Ad5VEGFE1 or Ad5VEGFLuc were injected into the left pleural space of nude mice. Mice were observed for 7 weeks for survival, and sacrificed to investigate intrapleural tumor formation microscopically.
To evaluate the suppression of pre‐established intrapleural tumors by the replication‐competent Ad in vivo, we injected the Ads into the intrapleural space in tumor‐bearing mice. Briefly, 2 weeks after intrapleural implantation of NCI‐H226 cells, 1 × 108 pfu of Ad5/3CMVLuc or Ad5/3VEGFE1 was injected directly into the pleural space, and then the mice were observed for survival.
Results
Transgene expression driven by VEGF promoter in the Ad context in vitro
In in vitro studies, we first evaluated five mesothelioma cell lines and three lung cancer cell lines for VEGF expression by RT‐PCR as reported previously.25 VEGF mRNA was expressed by all tested mesothelioma cell lines and lung cancer cell lines. Among four structural variants of VEGF (VEGF121, VEGF165, VEGF189, and VEGF206), VEGF121 and VEGF165 are the dominant isoforms in these cell lines as shown in Figure 2(a). These results indicated that mesothelioma cells produce VEGF proteins at high levels and suggested that the VEGF promoter in the Ad context could be activated in these cell lines. We next measured the activity of the VEGF promoter in an adenoviral context using a recombinant adenovirus expressing the luciferase gene (Ad5VEGFluc) in five mesothelioma cell lines in which VEGF mRNA expression was detected. In all tested lines infected by Ad5CMVLuc or Ad5/3CMVLuc, significant luciferase expression was confirmed by luciferase assay. Additionally, all mesothelioma cell lines were more susceptible to fiber‐modified Ad5/3 chimeric vector infection than to infection by the serotype 5 adenoviral vector based on a comparison of the luciferase activities of the cells infected by Ad5/3CMVLuc and Ad5CMVLuc. In these five mesothelioma cell lines infected by Ad5VEGFLuc, H226 showed 10–100 times higher luciferase expression than the other cell lines (Fig. 2b). Based on these data, we concluded that the VEGF promoter was able to induce transgene expression in VEGF‐producing mesothelioma cells and, notably, that the VEGF promoter retained its specificity when placed in the Ad genome.
Figure 2.
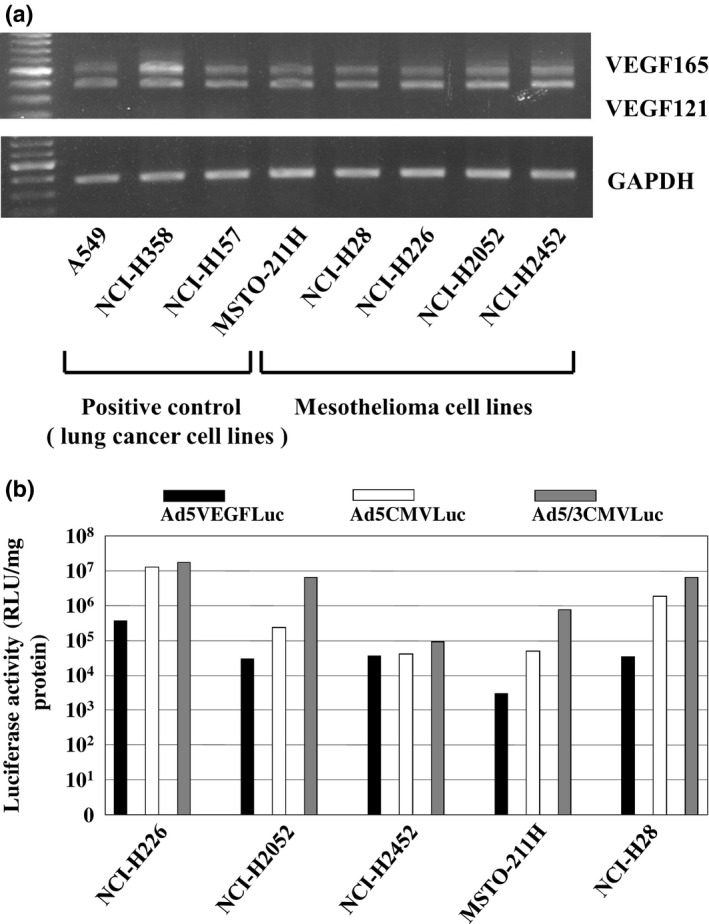
(a) Expression of VEGF mRNA in mesothelioma cell lines. A549, NCI‐H157, and NCI‐H358 are human lung cancer cell lines as positive control. MSTO‐211H, NCI‐H28, NCI‐H226, NCI‐H2052, and NCI‐H2452 are human malignant mesothelioma cell lines. Each RT‐PCR product for VEGF121 (408 bp) or VEGF165 (541 bp) is shown in the upper panel, and that for GAPDH (574 bp) is shown in the lower panel. The intensity of the RT‐PCR band corresponding to VEGF121 and VEGF165 was quantitated using an image analyzer. (b) Luciferase activity in mesothelioma cell lines infected with Ad5CMVLuc, Ad5VEGFLuc, or Ad5/3CMVLuc. 1 × 105 cells of each cell line were infected with each virus for 3 h at MOI 10. Cells were harvested and lysed in 100 μL of lysis buffer. 10 μL of each lysate was used for luciferase assay at 48 h after infection.
VEGF‐promoter‐driven CRAds replicate in vitro
To examine the replication of the replication‐competent Ads, NCI‐H226 cells were infected by Ad5VEGFE1 or Ad5/3VEGFE1, or Ad5/3CMVLuc as a negative control viral vector. We found that the replication‐competent Ads Ad5VEGFE1 and Ad5/3VEGFE1 produced progeny at significant levels at 3 and 6 days after infection as shown in Figure 3. Ad5/3VEGFE1 produced approximately 100 times more progeny than Ad5VEGFE1, probably because of the higher infectivity of Ad5/3 chimeric viral vector in NCI‐H226 cells. In contrast, the non‐replicative Ads, Ad5/3CMVLuc, did not produce any progeny despite have a strong promoter and a vector with high infectivity. Additionally, we previously demonstrated the inability of Ad5VEGFE1 to replicate in BEAS‐2B cells, which have low VEGF expression.27 These results indicate that the VEGF promoter retains its fidelity even in a replication‐competent adenoviral context and induces selective replication in VEGF‐producing mesothelioma cells, and further suggest that the VEGF‐promoter‐driven CRAds could also propagate in VEGF‐producing MPM tumors in vivo.
Figure 3.
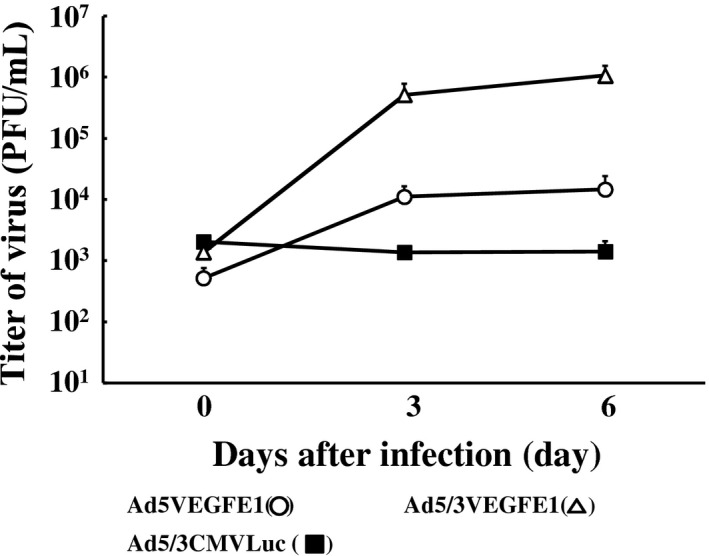
Assessment of viral replication in mesothelioma cells. NCI‐H226 cells (3 × 105) were infected with replication‐competent Ads (Ad5VEGFE1 [○] or Ad5/3VEGFE1 [∆]) or non‐replicative Ad (Ad5/3CMVLuc [■]) at an MOI of 10 for 3 h. Then, cells were divided into three portions and re‐plated in a 24‐well plate. Cells were harvested, and viral replication was assessed every 3 days in a standard plaque assay.
Specific cell‐killing efficacy of VEGF‐promoter‐driven CRAds
Next, we investigated the cell‐killing effect of replication‐competent Ads against to VEGF‐positive mesothelioma cell lines. In NCI‐H226 cells, Ad5VEGFE1 and Ad5/3VEGFE1 exerted cell‐killing effects as strong as those of Ad5CMVE1 as a positive‐control virus, as shown in Figure 4. All NCI‐H226 cells died by Day 12 after infection at an MOI of 0.1. In the other three mesothelioma cell lines, the cell‐killing effects were weaker than those shown in NCI‐H226 cells. The pattern observed in the cell‐killing results is different from that in the promoter activity assay because the viral infectivity is different in tumor cells.
Figure 4.
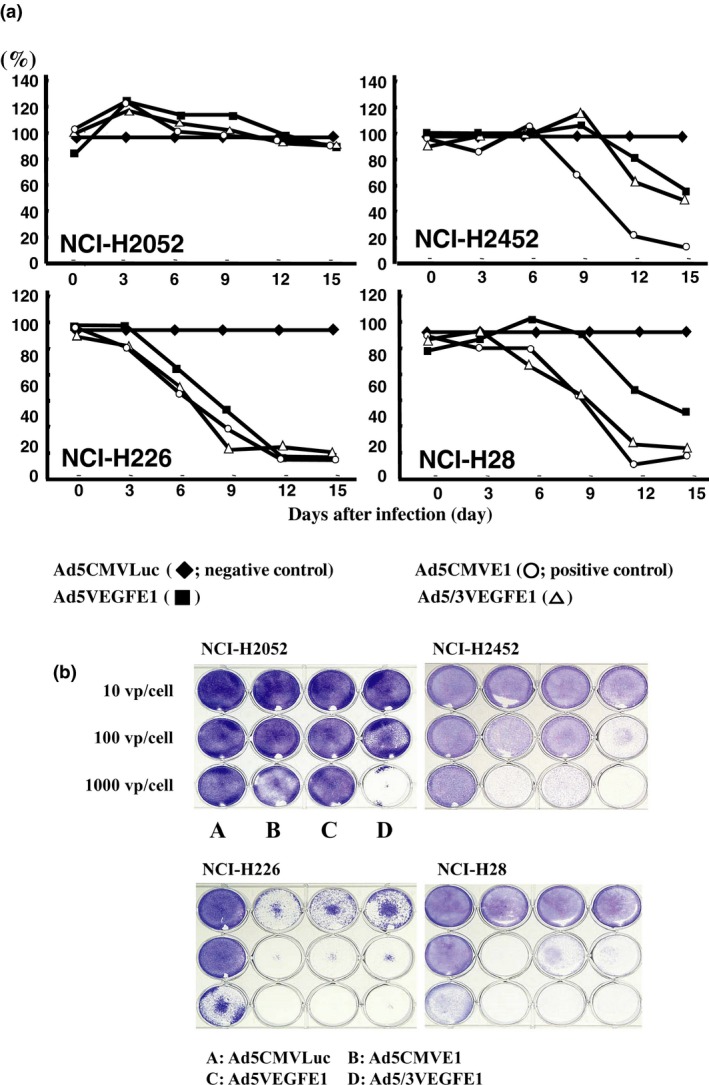
(a) The cell killing effect was evaluated by MTS assay. Mesothelioma cells (5 × 103) were infected with Ad5CMVLuc (◆; negative control), Ad5CMVE1 (○; positive control), Ad5VEGFE1 (■), or Ad5/3VEGFE1 (∆) at an MOI of 0.1. After infection, cell viability in each well was quantified by MTS assay every 3 days. The cell viability in each well was quantified by MTS assay every 3 days. The cell viability of cells infected with replication‐competent Ads (Ad5CMVE1, Ad5CMVE1, or Ad5/3VEGFE1) is expressed as the percentage of the OD490 value relative to control cells infected with Ad5CMVLuc (100%). (b) Cell killing effect was evaluated by crystal violet assay. Mesothelioma cells (2 × 105) were infected with each Ad (A: Ad5CMVLuc, B: Ad5CMVE1, C: Ad5VEGFE1, D: Ad5/3VEGFE1) at 10 vp/cell, 100 vp/cell, or 1000 vp/cell. All wells were stained by crystal violet to visualize the viable cells at 9 days after infection.
Tumor formation in nude mice
To investigate whether the VEGF‐promoter‐driven CRAds also exert an anti‐tumor effect in vivo, we first observed the intrapleural MPM tumor formation process in nude mice macroscopically and microscopically. We detected visible small tumors at 2 weeks after cell implantation, and the tumors then increased in size and invaded the surrounding lung, heart, or chest wall as shown in Figure 5. All mice (n = 3) harboring MPM tumors died within 50 days.
Figure 5.
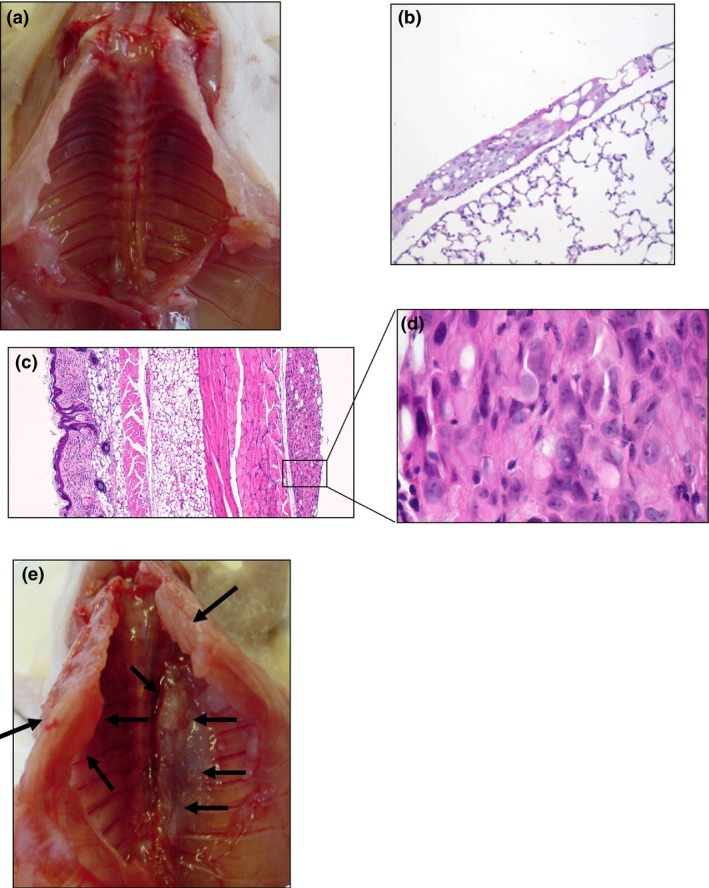
Tumor formation of malignant pleural mesothelioma (MPM) in nude mice. NCI‐H226 cells (1 × 107) were injected directly into left pleural space of nude mice. Mice were sacrificed every 2 weeks (a: 2 weeks, e: 4 weeks). Tumor were established on the bilateral lungs (b: 100×), pleural walls (c: 40×, d: 400×), diaphragms and pericardium at 2 weeks after implantation (HE staining).
Tumor growth suppression: pre‐infection with Ad5VEGFE1 in vitro
In the next step, we investigated the tumor‐suppression effect of the CRAds in vivo. In the control group, the mice were implanted in the pleural space with NCI‐H226 cells infected by Ad5VEGFLuc. Although the ratio of pre‐infected cells was as high as 50% of the total cells, significant tumor formation was shown in the pleural space (Fig. 6a). In contrast, mice implanted with NCI‐H226 cells including 10% cells pre‐infected with Ad5VEGFE1 showed no tumor growth in the pleural space (Fig. 6b). Furthermore, whereas all tumor‐bearing mice (n = 3) had died within 50 days in the control group, all mice (n = 3) in the treatment group survived in the observation period (Fig. 6c). These results suggest that pre‐infected NCI‐H226 cells produced progeny that spread and infect intact cells in the pleural space in vivo.
Figure 6.
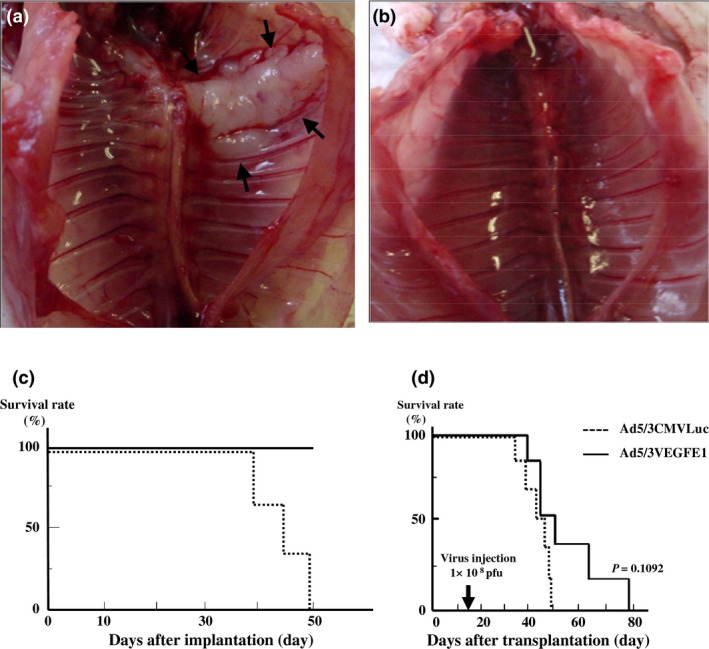
Tumor growth suppression: pre‐infection with Ad5VEGFE1 in vitro. NCI‐H226 cells (1 × 107) were pre‐infected with Ad5VEGFLuc (as control; a) or Ad5VEGFE1 (b) and mixed with non‐infected cells in various percentages before implantation. Mice were sacrificed at 7 weeks after implantation. (a) No effect of tumor suppression was seen in control mice (50% infected with Ad5VEGFLuc). (b) Tumor formation was suppressed when cells were infected with Ad5VEGFE1 (10% infected with Ad5VEGFE1). (c) Survival time of (a) or (b). (d) Tumor growth suppression by Ad5/3VEGFE1 in vivo. Two weeks after implantation of H226 cells (1 × 107), mice (n = 6) received a single injection of 1 × 108 pfu of Ad5/3VEGFE1. Control mice (n = 6) received the same dose of Ad5/3CMVLuc. Improvement of survival time by Ad5/3VEGFE1 was observed (P = 0.1092).
Tumor growth suppression by Ad5/3VEGFE1 in vivo
Lastly, we investigated whether replication‐competent Ads suppressed tumor growth after MPM tumors had been established. We injected Ad5/3VEGFE1 (1 × 108 pfu) directly into the left pleural space of the tumor‐bearing mice once at day 15 after implantation. The survival time in the group treated with Ad5/3VEGFE1 tended to be longer, although the difference between the survival curves of both groups was not significant, as shown in Figure 6(d) (P = 0.1092). These results suggest that Ad5/3VEGFE1 could potentially suppress MPM tumor growth even after tumor establishment in vivo.
Discussion
Malignant pleural mesothelioma incidence is increasing worldwide, and MPM is resistant to conventional chemotherapy with cytotoxic drugs and radiotherapy; therefore, the development of a new treatment strategy is urgent. Gene therapy is a promising modality for treatment because MPM tumors localize in the pleural space, which is easily accessible by percutaneous punctuation and has a large surface suitable for gene transduction by viral vectors. A phase I clinical trial for MM has already been conducted using a replication‐incompetent Ad expressing NK4 to inhibit the HGF/c‐Met pathway in Japan.28 However, one of the major disadvantages of treatment with a non‐replicative Ad is lower penetration of the viral vector in solid tumors. Thus, non‐replicative Ads transfer the gene only to the superficial layers of the tumor and are not able to infect the deep layers.29 In this regard, replication‐competent Ads might help to overcome the disadvantages of Ads and provide superior intratumoral penetration because of their ability to replicate and release new virus progeny.
In this study, we focused on higher VEGF expression in mesothelioma cells11 and conceived the application of VEGF promoter‐based conditionally replicative Ads, Ad5VEGFE1 or Ad5/3VEGFE1, for MPM treatment. As expected, these viral vectors were able to replicate in cultured mesothelioma cells, similarly to the replication in lung cancer cells that we reported previously.22 VEGF‐CRAd replication can be driven by the VEGF secreted from cancer cells and results in oncolytic effects. The virus does not reduce tumor angiogenesis by altering the function of tumor‐associated endothelial cells.
Although we did not demonstrate the replication of VEGF‐CRAd in vivo directly, our results suggest that newly produced VEGF‐CRAd progeny viruses from pre‐infected mesothelioma cells infect to intact mesothelioma cells in the intrapleural space in mice. In the vivo model, mesothelioma cells injected into the pleural space formed tumors of various shapes intrapleurally. Although the case in Figure 5 shows a flatly spreading tumor, some other mice showed a solid mass in the pleural space. The effectiveness of viral infection may depend on the surface area of the tumor, and flatly spreading tumors may be more susceptible to viral infection than solid masses. Therefore, we speculate that the two mice with a relatively longer survival time may have had such flatly spreading tumors, resulting in a better outcome for the viral therapy. Because all mice were observed until death, no clear difference in the pleural tumors was found macroscopically and histologically at the time of death, which is a limitation in this experiment. In the future, we plan to visualize the tumor formation process by computed tomography or by using a bioimaging tool before and after treatment. Recently, Zalcman et al.15 reported that addition of bevacizumab to pemetrexed plus cisplatin significantly improved OS for advanced malignant pleural mesothelioma in a randomized, controlled, open‐label, phase 3 trial. The result of this clinical trial highlighted the importance of blocking the VEGF signal pathway in the treatment of mesothelioma. From another perspective, mesothelioma expresses VEGF as a significant signal for tumor growth, which is also advantageous for the propagation of VEGF‐CRAd.
The safety profile, including the potential for an excessive immune response, is also a key consideration for clinical application of this treatment strategy. A previous study showed that one of the disadvantages of intrapleural instillation of recombinant Ads was that it resulted in mild microscopic pleuritis, myositis, and epicarditis, although these histological changes were restricted to the pleural space, were not associated with morbidity or mortality, and were reversible.30 Here, we investigated the intrapleural organs at 1 week after instillation of replication‐competent viruses and observed inflammatory changes in the pleural space, as reported previously. However, we detected no damage in the lung and pleura macroscopically and microscopically in this study (Fig. S1). Thus, in contrast to the inflammatory responses observed after high‐dose recombinant Ad administration into the lung,31, 32 the inflammatory changes resulting from intrapleural administration are not significant. A previous study also reported viral dissemination to extrathoracic organs after intrapleural administration, possibly as a result of hematogenous spread, but this was transient and not associated with clinical, pathologic, or biochemical abnormalities related to organ dysfunctions.30 If the Ads propagated in the pleural space leak into the extrathoracic space, the amount of leaked virus would be small, and also Ads driven by the VEGF promoter would not be activated in normal tissues and would disappear without any severe toxicity.
In previous studies, neutralizing antibodies in the serum and the pleural space developed following both intranasal33 and intrabronchial34 administration, but these immune responses induced no adverse clinical sequence.35 However, neutralizing antibodies in the pleural space might bind to the viral vector, limit gene‐transfer efficiency, and potentially negate the effects of viral therapy. Systemic corticosteroid administration has been shown to reduce clinical inflammatory responses but not inhibit antibody production;36 therefore, additional studies aimed at inhibiting antibody development, particularly in the pleural space, are required.
In summary, we have demonstrated that VEGF‐CRAd produces oncolytic effects in MM in vitro and offers survival benefits in MPM tumor‐bearing mice. Although statistical significance was not achieved and further investigation is required, we believe that the data we have reported here provide a basis for MM treatment with VEGF‐CRAd.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- CRAd
conditionally replicative adenovirus
- VEGF
vascular endothelial growth factor
- VP
viral particle
Supporting information
Fig. S1. HE staining of lungs following virus administrations.
Cancer Sci 108 (2017) 116–123
Funding Information
No sources of funding were declared for this study.
References
- 1. Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011; 89: 716–24, 24A–24C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Myojin T, Azuma K, Okumura J, Uchiyama I. Future trends of mesothelioma mortality in Japan based on a risk function. Ind Health 2012; 50: 197–204. [DOI] [PubMed] [Google Scholar]
- 3. Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009; 39: 576–88. [DOI] [PubMed] [Google Scholar]
- 4. Flores RM, Zakowski M, Venkatraman E et al Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007; 2: 957–65. [DOI] [PubMed] [Google Scholar]
- 5. Batirel HF, Metintas M, Caglar HB et al Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008; 3: 499–504. [DOI] [PubMed] [Google Scholar]
- 6. Aggarwal C, Somaiah N, Simon G. Antiangiogenic agents in the management of non‐small cell lung cancer: where do we stand now and where are we headed? Cancer Biol Ther 2012; 13: 247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis inhibition as a therapeutic strategy in non‐small cell lung cancer (NSCLC). Transl Lung Cancer Res 2015; 4: 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adachi Y, Aoki C, Yoshio‐Hoshino N, Takayama K, Curiel DT, Nishimoto N. Interleukin‐6 induces both cell growth and VEGF production in malignant mesotheliomas. Int J Cancer 2006; 119: 1303–11. [DOI] [PubMed] [Google Scholar]
- 9. Konig J, Tolnay E, Wiethege T, Muller K. Co‐expression of vascular endothelial growth factor and its receptor flt‐1 in malignant pleural mesothelioma. Respiration 2000; 67: 36–40. [DOI] [PubMed] [Google Scholar]
- 10. Strizzi L, Catalano A, Vianale G et al Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol 2001; 193: 468–75. [DOI] [PubMed] [Google Scholar]
- 11. Batra H, Antony VB. Pleural mesothelial cells in pleural and lung diseases. J Thorac Dis 2015; 7: 964–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, Sharma R. Inflammation‐based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 2012; 7: 587–94. [DOI] [PubMed] [Google Scholar]
- 13. Masood R, Kundra A, Zhu S et al Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF‐C autocrine loops. Int J Cancer 2003; 104: 603–10. [DOI] [PubMed] [Google Scholar]
- 14. Ceresoli GL, Zucali PA. Anti‐angiogenic therapies for malignant pleural mesothelioma. Expert Opin Investig Drugs 2012; 21: 833–44. [DOI] [PubMed] [Google Scholar]
- 15. Zalcman G, Mazieres J, Margery J et al Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open‐label, phase 3 trial. Lancet 2015; 387: 1405–14. [DOI] [PubMed] [Google Scholar]
- 16. Zhang W, Wu X, Wu L, Zhang W, Zhao X. Advances in the diagnosis, treatment and prognosis of malignant pleural mesothelioma. Ann Transl Med 2015; 3: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tada Y, Shimada H, Hiroshima K, Tagawa M. A potential therapeutic strategy for malignant mesothelioma with gene medicine. Biomed Res Int 2013; 2013: 572609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim KH, Dmitriev IP, Saddekni S et al A phase I clinical trial of Ad5/3‐Delta24, a novel serotype‐chimeric, infectivity‐enhanced, conditionally‐replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol Oncol 2013; 130: 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burke JM, Lamm DL, Meng MV et al A first in human phase 1 study of CG0070, a GM‐CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol 2012; 188: 2391–7. [DOI] [PubMed] [Google Scholar]
- 20. Takagi‐Kimura M, Yamano T, Tamamoto A et al Enhanced antitumor efficacy of fiber‐modified, midkine promoter‐regulated oncolytic adenovirus in human malignant mesothelioma. Cancer Sci 2013; 104: 1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Z, Jiang Q, Liu J et al A simplified system for generating recombinant E3‐deleted canine adenovirus‐2. Plasmid 2015; 77: 1–6. [DOI] [PubMed] [Google Scholar]
- 22. Takayama K, Reynolds PN, Adachi Y et al Vascular endothelial growth factor promoter‐based conditionally replicative adenoviruses for pan‐carcinoma application. Cancer Gene Ther 2007; 14: 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maizel JV Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 1968; 36: 115–25. [DOI] [PubMed] [Google Scholar]
- 24. Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 1996; 70: 7498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohta Y, Endo Y, Tanaka M et al Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res 1996; 2: 1411–6. [PubMed] [Google Scholar]
- 26. Adachi Y, Reynolds PN, Yamamoto M et al A midkine promoter‐based conditionally replicative adenovirus for treatment of pediatric solid tumors and bone marrow tumor purging. Cancer Res 2001; 61: 7882–8. [PubMed] [Google Scholar]
- 27. Takayama K, Ueno H, Nakanishi Y et al Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res 2000; 60: 2169–77. [PubMed] [Google Scholar]
- 28. Tada Y, Hiroshima K, Shimada H et al A clinical protocol to inhibit the HGF/c‐Met pathway for malignant mesothelioma with an intrapleural injection of adenoviruses expressing the NK4 gene. Springerplus 2015; 4: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev 2012; 32: 1078–91. [DOI] [PubMed] [Google Scholar]
- 30. Kucharczuk JC, Raper S, Elshami AA et al Safety of intrapleurally administered recombinant adenovirus carrying herpes simplex thymidine kinase DNA followed by ganciclovir therapy in nonhuman primates. Hum Gene Ther 1996; 7: 2225–33. [DOI] [PubMed] [Google Scholar]
- 31. Chen RF, Lee CY. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. Int Rev Immunol 2014; 33: 45–53. [DOI] [PubMed] [Google Scholar]
- 32. Brody SL, Metzger M, Danel C, Rosenfeld MA, Crystal RG. Acute responses of non‐human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther 1994; 5: 821–36. [DOI] [PubMed] [Google Scholar]
- 33. Zabner J, Petersen DM, Puga AP et al Safety and efficacy of repetitive adenovirus‐mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet 1994; 6: 75–83. [DOI] [PubMed] [Google Scholar]
- 34. Joseph PM, O'Sullivan BP, Lapey A et al Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther 2001; 12: 1369–82. [DOI] [PubMed] [Google Scholar]
- 35. Molnar‐Kimber KL, Sterman DH, Chang M et al Impact of preexisting and induced humoral and cellular immune responses in an adenovirus‐based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther 1998; 9: 2121–33. [DOI] [PubMed] [Google Scholar]
- 36. Sterman DH, Molnar‐Kimber K, Iyengar T et al A pilot study of systemic corticosteroid administration in conjunction with intrapleural adenoviral vector administration in patients with malignant pleural mesothelioma. Cancer Gene Ther 2000; 7: 1511–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. HE staining of lungs following virus administrations.


