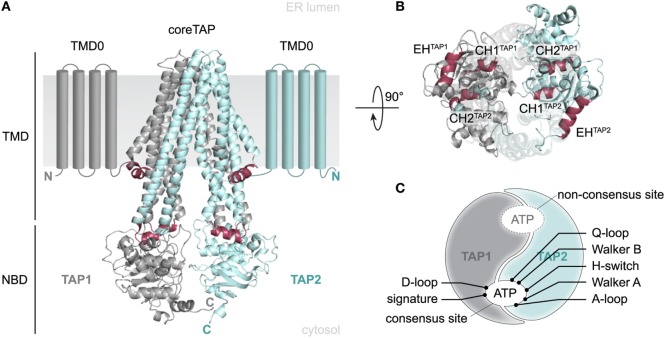
Figure 1.
Structural organization of the antigen translocation complex transporter associated with antigen processing (TAP). (A) 3D homology model of the human TAP complex based on the TAP-related heterodimeric ABC-binding cassette transporter TmrAB in the inward-facing conformation (13, 14). The heterodimeric translocation machinery (TAP1 and TAP2) consists of a 2 × 6 TMHs core domain, two additional N-terminal four-transmembrane helix bundles (TMD0, schematically shown), and two nucleotide-binding domains (NBDs). Core transmembrane domains (TMDs) and TMD0s are connected via elbow helices (EHs). Two NBDs facilitate ATP binding and hydrolysis. (B) Top view from the ER lumen along the TMD–NBD interface. Coupling helix CH1 mediates inter-domain cross talk between NBDs and TMDs in cis and in trans, whereas CH2 interacts in trans. (C) Schematic view of the asymmetric NBDs of the TAP complex. NBDs are tightly packed in the outward-facing conformation and thus form two ATP-binding sites. The presence of non-equivalent, consensus and non-consensus ATPase sites is based on aberrant amino acid residues within the conserved sequence motifs. TAP1: gray, TAP2: blue, CH/EH: raspberry.