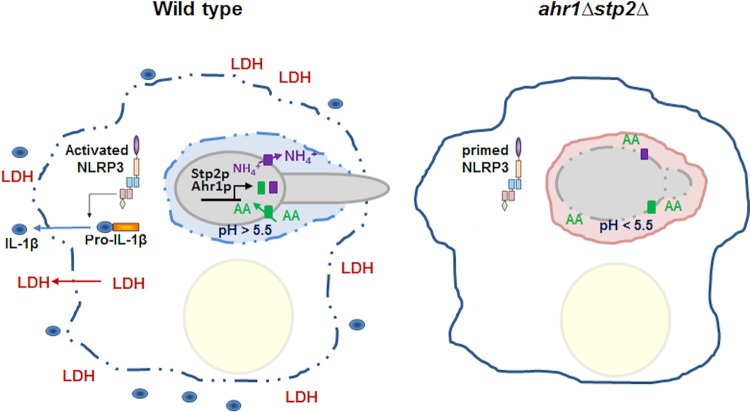
FIG 6.
Phagosomal neutralization triggers C. albicans-driven macrophage pyroptosis. Upon interaction with macrophages, C. albicans cells trigger activation of the NLRP3 inflammasome and expression of pro-IL-1β. Within the phagosome, the fungal cell takes up amino acids (AA), metabolizes them, and expels NH4+ into the phagosomal milieu, a process that is dependent on the transcription factors Stp2p and Ahr1p. This results in neutralization of the phagosome and hyphal morphogenesis, with the latter contributing to membrane distention and the loss of immune cell integrity. LDH and the proinflammatory cytokine IL-1β are released from the cell and serve as a danger signal to uninfected macrophages to react to infection. Most of the phagocytosed cells defective in pH modulation, such as cells of the ahr1Δ stp2Δ mutant, reside in acidic phagosomes in yeast form, fail to activate the inflammasome, and are cleared more readily by the macrophages. These cells restore the ability to form hyphae and to damage the immune cell upon pharmacological neutralization of the phagosome with the vATPase inhibitor bafilomycin A.