ABSTRACT
We previously reported that the Tet38 efflux pump is involved in internalization of Staphylococcus aureus by A549 lung epithelial cells. A lack of tet38 reduced bacterial uptake by A549 cells to 36% of that of the parental strain RN6390. Using invasion assays coupled with confocal microscopy imaging, we studied the host cell receptor(s) responsible for bacterial uptake via interaction with Tet38. We also assessed the ability of S. aureus to survive following alkalinization of the phagolysosomes by chloroquine. Antibody to the scavenger receptor CD36 reduced the internalization of S. aureus RN6390 by A549 cells, but the dependence on CD36 was reduced in QT7 tet38, suggesting that an interaction between Tet38 and CD36 contributed to S. aureus internalization. Following fusion of the S. aureus-associated endosomes with lysosomes, alkalinization of the acidic environment with chloroquine led to a rapid increase in the number of S. aureus RN6390 bacteria in the cytosol, followed by a decrease shortly thereafter. This effect of chloroquine was not seen in the absence of intact Tet38 in mutant QT7. These data taken together suggest that Tet38 plays a role both in bacterial internalization via interaction with CD36 and in bacterial escape from the phagolysosomes.
KEYWORDS: CD36, S. aureus, endosomes, internalization, survival
INTRODUCTION
Staphylococcus aureus is a versatile bacterium capable of causing acute and chronic infections in humans and animals due to its arsenal of virulence factors and its ability to acquire multiple drug resistance phenotypes (1–3). Chronic infections caused by S. aureus, such as osteomyelitis, are difficult to treat, and it and other staphylococcal infections are notable for their propensity to recur after treatment (4–6). This persistence may be due in part to the ability of S. aureus to survive in and adapt to the host intracellular environment, enabling escape from the effect of antibiotic treatment and the host immune response (7–10). Although S. aureus is not a traditional intracellular pathogen, many studies have demonstrated that it can invade and survive within nonprofessional phagocytic host cells, such as epithelial and endothelial cells (9, 11). In the case of Listeria monocytogenes, the internalization process starts with a molecular interaction between a bacterial surface protein and a host cell receptor, which leads to a series of signal inductions across the cell membrane, resulting in the rearrangement of the host cell cytoskeleton and finally internalization of the bacteria (12). S. aureus expresses a number of extracellular matrix proteins, termed microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), including fibronectin-binding proteins (FnBPs), which bind the heat shock protein Hsp60 of the host cell, the iron-regulated surface determinant B (IsdB), which interacts with host cell integrins, and lipoteichoic acids (LTAs), which are recognized by the Toll-like receptor TLR2/TLR6 dimers (13–15). The host cell receptor CD36 is a membrane glycoprotein of the class B scavenger family that interacts with Toll-like receptors TLR2 and TLR6 acting as a facilitator in the recognition of diacylglyceride components of bacteria. CD36 is also a long-chain fatty acid transporter present on the surface of epithelial and endothelial cells, as well as in intracellular compartments such as endosomes (16, 17). In a recent study of myocardial fatty acid uptake, Glatz et al. demonstrated that CD36 is able to translocate between the endosomes and the sarcolemma, enabling the transport of fatty acids to different intracellular locations and thereby playing an important role in the coordination of cardiac fatty acid uptake to meet myocardial energy needs (18). As a scavenger receptor, this protein can also recognize and internalize apoptotic cells, pathogenic fungi, and bacteria such as Escherichia coli, mycobacteria, and S. aureus (17–19). CD36 was reported as a phagocytic receptor for S. aureus that internalized this bacterium together with its LTA via the COOH-terminal cytoplasmic portion of CD36 (20).
Tet38 is an S. aureus efflux pump that can extrude both tetracycline and unsaturated free fatty acids, such as palmitoleic acid and undecanoic acid (21, 22). Tet38 plays an important role in bacterial colonization and internalization, but the mechanism of this involvement has not been explored. After internalization and fusion with lysosomes, depending on the cell lines, S. aureus can replicate rapidly and escape from the phagolysosome or persist for a time and escape later. In both circumstances, S. aureus produces alpha-toxin that induces cell apoptosis (23, 24). Recent studies by Leimer et al. showed that acidic pH induced nonstable S. aureus small-colony variants (SCVs) and nonreplicating persister cells that were localized to the phagolysosome. These SCVs were eliminated after alkalinization of the acidic milieu of the phagolysosome with chloroquine or other lysomotropic alkalinizing agents (25). Chloroquine diffuses freely and rapidly across cell membranes and accumulates in lysosomes in its unprotonated form. In the acidic environment of lysosomes (pH 4.5), chloroquine becomes protonated and is trapped in the acidic compartment (26).
In the present study, we evaluated the role of Tet38 in adherence, internalization, and intracellular trafficking in epithelial cells. We found that in the absence of Tet38 there was loss of the dependence of internalization on CD36, suggesting an interaction between Tet38 and CD36. Following the fusion of the S. aureus-associated endosomes with lysosomes, alkalinization of the acidic environment with chloroquine led to a rapid increase in the number of bacteria in the cytosol followed by a decrease shortly thereafter. This effect of chloroquine was also dependent on intact Tet38.
RESULTS
Interplay of host cell CD36 and the S. aureus Tet38 efflux pump contributes to efficient internalization of S. aureus by A549 cells.
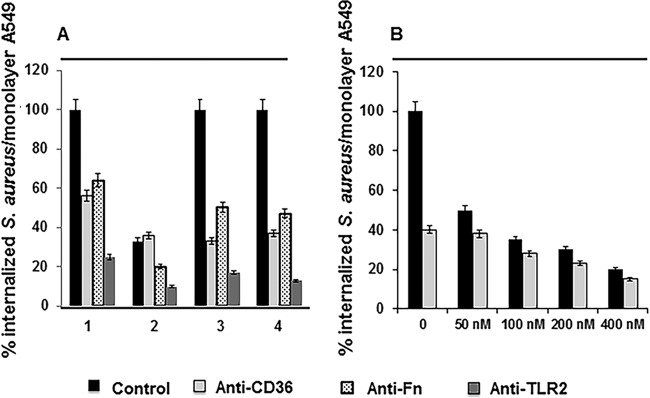
As we reported previously (22), Tet38 contributes to internalization of S. aureus by A549 cells. QT7 tet38 was internalized 6-fold less in A549 and human microvascular endothelial cells (HMECs) than the parent strain RN6390. The membrane-associated host cell receptor CD36 is a transporter of long-chain fatty acids and is also known to contribute to S. aureus invasion of host cells (18, 27). To determine if CD36 is the host cell ligand with which Tet38 interacts in the internalization process, we tested the effect of anti-CD36 antibody on internalization of RN6390 and QT7. We treated A549 epithelial cell monolayers with anti-CD36 antibody (50 nM) for 30 min prior to infection by RN6390 or QT7. After 1 h of contact, the monolayers were washed and incubated with assay medium containing gentamicin and lysostaphin to eliminate extracellular bacteria. All values (in percentages) were based on the value of the total internalization (100%) of RN6390 by A549. In the absence of anti-CD36 antibody, the amount of QT7 (tet38 mutant) internalized was 38% of the amount of internalized RN6390 (Fig. 1A). These data were similar to those reported in our previous study (22). After the monolayers were preincubated with anti-CD36 antibody at 50 nM, the amount of internalized wild-type RN6390 was reduced to 56% of the amount in the assay without CD36 antibody preincubation. In contrast, there was little or no reduction in internalization of QT7 (36% versus 38%) after CD36 antibody preincubation. To compare the effect of CD36 with that of a known host cell ligand, fibronectin, we also carried out the internalization assays after pretreatment with anti-fibronectin (anti-Fn) antibody. After preincubation with 50 nM antibody, RN6390 internalization decreased to 64%, and QT7 showed an additional decrease from 36% to 20% of the RN6390 level, representing a similar relative reduction to 55% of its value in the absence of anti-CD36. Thus, the effect of anti-CD36 antibody but not anti-fibronectin antibody on S. aureus internalization was reduced in the absence of Tet38.
FIG 1.
Effect of anti-CD36, anti-Fn (fibronectin), and anti-TLR2 (Toll-like receptor 2) antibodies on internalization of S. aureus RN6390 and QT7 by A549 cells. (A) Invasion assays using S. aureus RN6390, QT7, and the tet38-complementing transformants, with anti-CD36, anti-Fn, and anti-TLR2 antibodies at 50 nM. Lane 1, RN6390; lane 2, QT7 (tet38 mutant); lane 3, RN6390(pLZ113-tet38); lane 4, QT7(pLZ113-tet38). (B) Invasion assays using S. aureus RN6390 (black bars) and QT7 (gray bars) with anti-CD36 antibody at increasing concentrations from 0 nM to 400 nM. The A549 cell monolayers were preincubated for 30 min with antibody diluted to 50 nM (A) or the indicated concentrations (B). The number of bacteria per monolayer was determined following a standard internalization assay with an MOI of 100:1. The results are presented as a percentage of the CFU count/monolayer of the treated assay versus that of the nontreated assay, with the RN6390 CFU count/monolayer as the reference point for all assays (100%). All values are means ± standard deviations of three independent experiments. The differences in the numbers of bacteria recovered in each assay with and without treatment with antibodies are statistically significant as determined by Student's t test (P < 0.05).
Since the Toll-like receptor TLR2 was shown to form a complex with CD36, we carried out a similar invasion assay using anti-TLR2 antibody to assess the effect of this receptor on S. aureus internalization by A549 in the absence of tet38. After preincubation with 50 nM antibody, RN6390 internalization decreased to 25%, and QT7 showed an additional decrease from 36% to 10% of the RN6390 level, representing a relative reduction to 28% of its value in the absence of anti-CD36. These data indicated that TLR2 had its own bacterial target and also confirmed that the effect of anti-CD36 antibody was reduced in the absence of Tet38 (Fig. 1A).
Dose titrations of anti-CD36 antibody from 50 nM to 400 nM revealed that the progressively higher concentrations of anti-CD36 antibody had increasing effects on S. aureus internalization for both RN6390 and QT7 tet38 although the effect on QT7 remained attenuated relative to that on RN6390, with a reduction of 2.7-fold (15% versus 40%) for QT7 and 5-fold (20% versus 100%) for RN6390 in comparison to the results with no anti-CD36 antibody and those with the highest concentration, 400 nM (Fig. 1B). In addition, in internalization assays with Jurkat cells, which lack CD36, there was no difference between internalization of RN6390 and QT7. Both strains were internalized into Jurkat cells at 50% efficiency compared to that of RN6390 in A549 cells. Internalization values remained unchanged when anti-CD36 antibody was added to the Jurkat cell monolayer for 30 min prior to infection by either bacterial strain. In contrast, following preincubation with anti-Fn antibody, both RN6390 and QT7 showed similar levels of reduction from 50% (no preincubation) to 36% internalization into Jurkat cells (data not shown). Thus, these data suggest that an interaction between Tet38 and CD36 contributes to the internalization of S. aureus in A549 epithelial cells. Since CD36 forms complexes with other host cell receptors, such as TLR2/TLR6, it is possible that the interactions with Tet38 also involve other proteins, interactions that could be disrupted at the highest concentrations of anti-CD36.
Dose titrations of anti-Fn antibody from 50 nM to 400 nM confirmed that the roles of fibronectin-binding proteins in S. aureus internalization were similar in the presence or absence of Tet38, with anti-Fn providing a reduction of 64% to 25% for RN6390 and of 20% to 10% for QT7 at the highest antibody concentration (400 nM) (data not shown).
The tet38-overexpressor RN6390(pLZ113-tet38) showed a similar level of internalization in A549 cells as that of RN6390 alone, suggesting that expression of tet38 in RN6390 may be a maximal level for interaction with CD36 in A549 cells. Invasion assays carried out using the tet38-complementing strain QT7(pLZ113-tet38) yielded the same level of internalization as that of RN6390 (100%) in the control assay. Figure 1A represents the internalization data of all S. aureus strains as percentages of the internalization of RN6390.
Effect of Tet38 on interaction of S. aureus with host cell membranes.
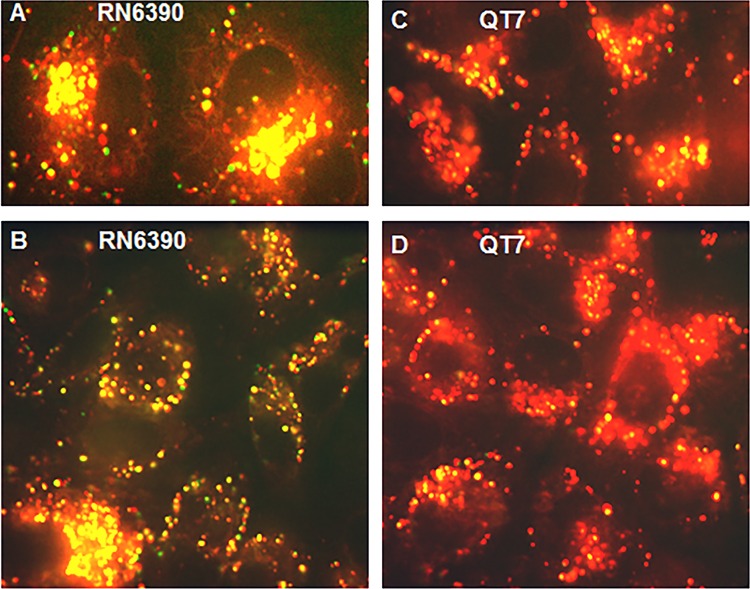
To identify further the events occurring at the S. aureus-A549 cell membrane interaction, we carried out confocal microscopy imaging to visualize the association between bacteria and host cell membranes. We stained the A549 cells with the dye FM4-64, which labels the plasma membrane (red) and various organelles except the nucleus and used yellow fluorescent protein (YFP)-labeled RN6390 and QT7 for the infection (Fig. 2). S. aureus was added to A549 cells for 2 h, followed by incubation of the monolayers with assay medium containing gentamicin and lysostaphin for 60 min to eliminate remaining extracellular bacteria. Under these conditions, bacteria not associated with cell membrane structures fluoresce green, and those colocalized with the cell membrane (endosomes) fluoresce yellow. We enumerated green and yellow bacteria in 10 contiguous confocally imaged fields, each with an average of six A549 cells. Overall, as expected, there were fewer A549-associated bacteria (yellow) for QT7 (34%) than A549-associated bacteria (yellow) for RN6390 (considered as 100%) (Fig. 3). Preincubation of A549 cells with anti-CD36 antibody resulted in a 2-fold reduction (100% to 50%) in cell-associated RN6390 but no reduction in cell-associated QT7 (1.03-fold, or 34% to 33%). In contrast, anti-fibronectin antibody resulted in similar reductions for cell-associated RN6390 and QT7 (1.7-fold, or 100% to 58%, and 1.4-fold, or 34% to 25%, respectively). There was little or no effect of either antibody on the number of remaining non-cell-membrane-associated bacteria (green). Thus, internalization of S. aureus in A549 cell membranes is also affected by blocking CD36 in a Tet38-dependent manner (Fig. 3).
FIG 2.
Confocal microscopy of S. aureus RN6390(pAH16) and QT7(pAH16) after 2 h of internalization following a 30-min treatment with lysostaphin and gentamicin in DMEM. A549 lung epithelial cells were stained with FM4-64 (red). The bacteria expressed YFP and were viewed as green fluorescence. Colocalization of S. aureus and A549 was viewed as yellow fluorescence. Representative fields are shown.
FIG 3.
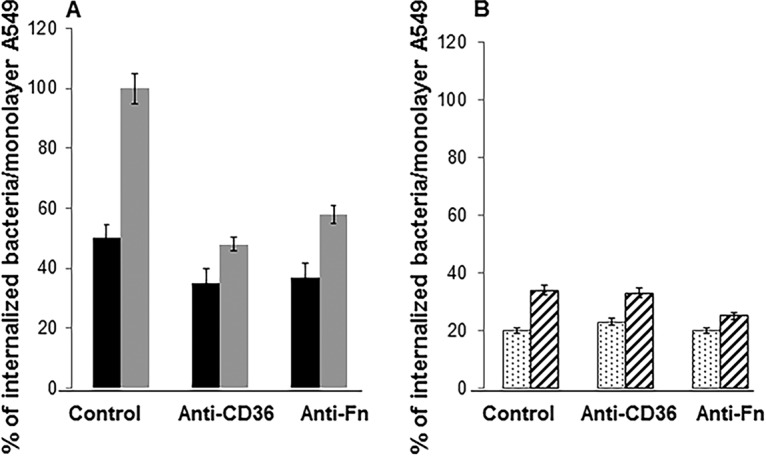
Quantification of the association of internalized S. aureus RN6390 (A) and QT7 (tet38) (B) and the A549 cell membrane using confocal microscopy. A549 cells were stained with the dye FM4-64. YFP-expressing bacteria (green) and host cells (red) were in contact for 2 h, followed by an additional 30 min in medium containing gentamicin and lysostaphin. We counted the numbers of green fluorescent bacteria (nonassociated bacteria) and yellow fluorescent bacteria (associated bacteria) from 60 A549 cells of 10 fields. The percentage of internalized bacteria was calculated based on the total number of green or yellow fluorescent bacteria counted from treated assays versus that of nontreated assays. The total number of RN6390 bacteria in nontreated assays was used as the reference point (100%). The assays were done in triplicate. The differences in the numbers of bacteria recovered in each assay with and without treatment with antibodies are statistically significant as determined by Student's t test (P < 0.05). Black bars in panel A represent nonassociated green cells, and gray bars represent yellow cells associated with A549 membrane. Dotted bars in panel B represent nonassociated green cells, and striped bars represent yellow cells associated with A549 membrane.
Involvement of Tet38 in S. aureus trafficking within A549 cells.
We next examined the trafficking of internalized S. aureus with phagolysosomes, which are formed by the fusion of S. aureus-associated endosomes with the lysosomes of the A549 host cell. For these experiments, we extended the period of incubation of A549 cells after internalization of S. aureus out to 4 days, stained the lysosomes of A549 using the LysoTracker dye DND99 (red), and used the YFP-expressed RN6390 and QT7 (green) to localize cells after internalization. We observed the colocalization of the internalized bacteria/lysosomes (yellow) over time using confocal microscopy (Fig. 4). Both free RN6390 and bacteria associated with acidic lysosomes increased over 3 days and then decreased, with somewhat fewer phagolysosome-associated bacteria than free bacteria throughout that time period (Fig. 5A). In contrast, for QT7, there were 2- to 3-fold more cells associated with the phagolysosome than free cells on days 1 and 2, followed by decreases in phagolysosome-associated cells without an increase of free cells and with similar numbers of free and phagolysosome-associated cells by day 4 (Fig. 5B).
FIG 4.
Confocal microscopy of S. aureus RN6390(pAH16) and QT7(pAH16) at 4 h after addition of lysostaphin and gentamicin in DMEM. The lysosomes of the lung epithelial cells A549 were stained with LysoTracker DND99 (red). The bacteria expressed YFP and were viewed as green cells. Colocalization of S. aureus and A549 was viewed as yellow cells. Representative fields are shown.
FIG 5.

Quantification of the association between internalized S. aureus strains RN6390 and QT7 (tet38) and A549 phagolysosomes using confocal microscopy. A549 cells were stained with the dye LysoTracker DND99. YFP-expressing bacteria (green) and lysosomes (red) were in contact for 4 h following an additional 30 min in medium containing gentamicin and lysostaphin. The assays were carried out in the presence or absence of chloroquine (20 μM). We counted the numbers of green cells (nonassociated bacteria) and yellow cells (associated bacteria) from 60 A549 cells of 10 images (number of CFU/60 A549 cells). The assays were done in triplicate. The differences in RN6390 and QT7 bacterial cells treated or not treated with chloroquine are statistically significant as determined by Student's t test (P < 0.05). In both panels, the symbols are as follows: black line, green cells, nonassociated with lysosomes, without chloroquine; dashed black line, green cells, nonassociated with lysosomes, plus chloroquine; gray line, yellow cells, associated with lysosomes, without chloroquine; dashed gray line, yellow cells, associated with lysosomes, plus chloroquine.
Effect of chloroquine on the survival and localization of S. aureus in A549 cells.
Chloroquine is taken up by A549 and other epithelial cells and is concentrated in the phagolysosome, causing an increase in its pH (25). To assess the effect of phagolysosomal pH on the localization patterns of RN6390 and QT7, tracking experiments were repeated in the presence of chloroquine (20 μM) in a manner similar to that previously reported (13). Chloroquine had similarly limited effects on growth of both RN6390 and QT7 in vitro, and the two strains did not differ in their inabilities to grow in vitro at pH 4.5 (Fig. 6A), the pH of the phagolysosome (26). Within A549 cells, however, survival of RN6390 was increased over 2-fold in the presence of chloroquine, with no apparent enhancement of QT7 growth (Table 1; Fig. 6B). Thus, Tet38 appears to enable increased intracellular growth upon increase of pH in the phagolysosome.
FIG 6.
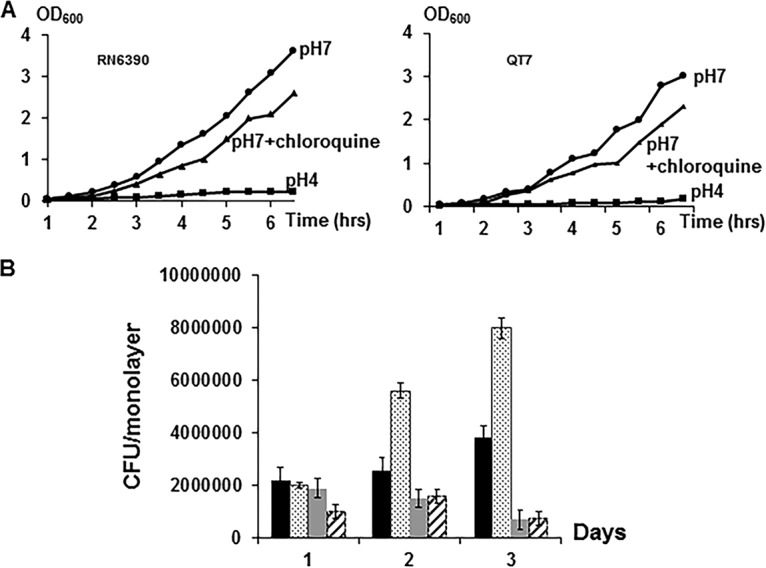
Growth curves of S. aureus in different media. (A) Growth in vitro. (B) Internalization of S. aureus in A549 cells. The effect of chloroquine treatment is shown. Black bars, RN6390; gray bars, QT7 (tet38); dotted bars, RN6390 with chloroquine; striped bars, QT7 (tet38) with chloroquine. The assays were done in triplicate. The differences in CFU of RN6390 and QT7 per monolayer recovered in each assay with and without treatment with chloroquine are statistically significant as determined by Student's t test (P < 0.05).
TABLE 1.
Internalization of S. aureus by A549 cells
| Time point (day) |
S. aureus internalization by strain and condition (CFU/monolayer)a |
|||
|---|---|---|---|---|
| RN6390 |
QT7 |
|||
| − Chloroquine | + Chloroquine | − Chloroquine | + Chloroquine | |
| 1 | (2.2 ± 0.005) × 106 | (2.0 ± 0.001) × 106 | (1.9 ± 0.001) × 106 | (1.0 ± 0.001) × 106 |
| 2 | (2.6 ± 0.003) × 106 | (4.6 ± 0.002) × 106 | (1.5 ± 0.005) × 106 | (1.2 ± 0.002) × 106 |
| 3 | (3.8 ± 0.005) × 106 | (8.0 ± 0.001) × 106 | (7.0 ± 0.002) × 105 | (7.5 ± 0.002) × 105 |
| 4 | (7.2 ± 0.02) × 103 | (5.6 ± 0.01) × 103 | (2.1 ± 0.02) × 103 | (9.5 ± 0.2) × 102 |
| 5 | 300 ± 10 | 250 ± 20 | 90 ± 5 | 50 ± 2 |
| 6 | 150 ± 5 | 100 ± 10 | 25 ± 5 | 10 ± 1 |
Experiments were done in triplicate and with three separate biological samples with (+) and without (−) chloroquine. The differences in RN6390 and QT7 bacterial cells treated or not treated with chloroquine are statistically significant as determined by a Student's t test (P < 0.05).
We next evaluated the effect of chloroquine on intracellular trafficking of RN6390 and QT7 (Fig. 5). For RN6390 chloroquine resulted in a decrease in phagolysosome-associated bacteria and a substantial increase in free bacteria, reaching a peak of approximately 2-fold at day 3. In contrast, chloroquine had little effect on QT7 intracellular localization, with no change in free bacteria and a slight decrease in phagolysosome-associated bacteria. Thus, Tet38 enables escape from or avoidance of the phagolysosome associated with increased phagolysosomal pH, an effect that may underlie the increased intracellular survival seen with RN6390 in the presence of chloroquine.
DISCUSSION
We previously demonstrated that S. aureus RN6390 internalized efficiently into the A549 lung epithelial cells (22) and that this ability was reduced in the absence of Tet38, a proton antiporter efflux pump that is capable of transporting tetracycline and certain antibacterial fatty acids (palmitoleic acid and undecanoic acid) but whose specific role in bacterial invasion and intracellular survival is not well understood (21). We thus undertook to evaluate directly the role of Tet38 in S. aureus in adherence and internalization for epithelial cells and its effect on trafficking within the host cells.
A number of S. aureus surface proteins are known to mediate adherence to host cells, including fibronectin-binding proteins A and B (FnbAB), elastin-binding proteins A and B (EbhAB), extracellular adhesion protein (Eap), protein A, autolysin (Atl), and other proteins collectively referred to as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules). These proteins recognize and adhere to the extracellular matrix components of the host cells, such as fibronectin, fibrinogen, α5β1 integrins, heat shock proteins Hsp60 and Hsp70, and various other receptors (28–31). Among host cell receptors, CD36 plays a major role in S. aureus internalization. CD36 is a member of the scavenger receptor family and is involved in the uptake of long-chain fatty acids by myocardial cells. CD36 receptors regularly cycle between the myocardial cell surface pools and endosomes (18). In certain cell lines such as the HeLa cells, overexpression of CD36 leads to an increase in S. aureus uptake. This capacity to facilitate bacterial uptake was demonstrated by lysosomal colocalization of E. coli K-12 and CD36 protein within CD36-overexpressing HeLa cells (17). In addition, there is a reduction in E. coli and S. aureus internalization and survival in CD36-deficient phagocytes and in mice deficient in class B scavenger receptors, such as CD36 and SR-BI II (16). CD36 is thought to form a cluster complex with Toll-like receptors TLR2 and TLR6 in response to diacylated lipopeptides. As a component of the host innate immunity, TLR2 recognizes teichoic acids exposed on the surface of the S. aureus cell wall. Furthermore, CD36 was reported as a selective and nonredundant sensor of microbial diacylglycerides that signal via the TLR2/TLR6 heterodimer (32). Thus, we tested the hypothesis that Tet38 is a bacterial protein that interacts with CD36 in mediating internalization of S. aureus into epithelial cells. Tet38 is an efflux pump located in the cell membrane of S. aureus. It is predicted from hydropathy plots to have only small extracellular domains. Thus, to be able to interact with a host cell component such as CD36, Tet38 may need an intermediate partner such as the LTA to establish the contact. The hypothesis regarding the connection between Tet38 and LTA is under investigation. Our findings document that anti-CD36 antibody specifically reduces internalization of S. aureus in A549 cells and that the effect of antibody is either eliminated or attenuated in an otherwise isogenic tet38 mutant. Such attenuation in a tet38 mutant is not seen using antibody to fibronectin, a known target of S. aureus fibronectin-binding protein, or antibody to TLR2, supporting the specificity of the interaction of Tet38 with CD36. In addition, the reduced S. aureus internalization seen in Jurkat cells, which lack CD36, is not dependent on the presence of Tet38. Our data thus strongly suggest an interaction between Tet38 and CD36, probably in an indirect manner due to the location of Tet38 within the bacterial membrane. Because high concentrations of anti-CD36 antibody can somewhat reduce internalization further in Tet38-deficient cells, it is possible that CD36 also has additional bacterial ligands or that high antibody concentrations can affect other coreceptor complex members such as TLR2 and its binding to lipoteichoic acids. Internalization assays using anti-TLR2 antibody showed that the absence of Tet38 in mutant QT7 did not affect the ability of TLR2 to interact with other bacterial components, suggesting that TLR2 has a specific staphylococcal ligand(s) that is different from Tet38. Additional binding studies are ongoing.
Endosome-internalized S. aureus bacteria are known to traffic to the phagolysosome, which maintains a growth-inhibitory acid pH (26). Since the tet38 mutant fails to replicate within A549 cells and since replication of wild-type cells is delayed (which is possibly related to the time of the bacteria's release from the phagolysosome), we postulated that Tet38 may also affect intracellular trafficking. Using fluorescently labeled S. aureus and a lysosome-specific dye with confocal microscopy, we could localize intracellular bacteria within and outside the phagolysosome over the course of several days. Intracellular tet38 mutant bacteria remained dominantly associated with the phagolysosome over 2 days, with a progressive decrease in numbers thereafter (Fig. 5), which temporally correlated with a failure to replicate, followed by a reduction in viable cells (Fig. 6B). In contrast, wild-type intracellular bacteria with intact Tet38 consistently had greater numbers of cells not associated with the phagolysosome than associated with it, correlating with increasing numbers of viable cells over time. These findings suggest that in the absence of Tet38, bacteria become trapped in the phagolysosome, and viable cells are thereby reduced.
Acidification of the phagolysosome is thought to be an important component of the ability of host cells to control survival of intracellular bacteria (25). It has been shown that acidification of the phagosomes was dependent on the movement of protons mediated by a vacuolar-type H+-ATPase across the phagosomal membrane. This acidification could be selectively blocked by bafilomycin A1, a macrolide antibiotic, or by the antimalaria drug chloroquine (25, 33). Chloroquine diffuses into cells and concentrates in phagolysosomes, raising their pH (26). We determined the role of acid pH in the differences between wild-type and tet38 mutant intracellular bacteria by assessing the effect of chloroquine on cell viability and trafficking in A549 cells. As seen in other studies, reduced acidification of the phagolysosome with chloroquine increased both viability and the escape of wild-type cells from the phagolysosome (25). In contrast, reduced acidification of the phagolysosome had little or no effect on the viability of the intracellular tet38 mutant or its remaining dominantly associated with the phagolysosome. Thus, Tet38 enables further escape from the phagolysosome when acidification is decreased. The mechanism of this effect is uncertain. Tet38 is a member of the major facilitator superfamily (MFS) which includes proton antiporters and is capable of extruding tetracycline and certain fatty acids (22). If Tet38 played a role in exchanging efflux substrates such as antibacterial fatty acids for uptake of protons, higher external proton concentrations (acidity in phagolysosome versus bacterial cell) would be expected to reduce its efflux activity and presumably also reduce its ability to protect against antibacterial fatty acids or other unknown substrates. In this case, Tet38 would not be beneficial to the bacterial cell in this acidic environment, leading to downregulation of the transcription of tet38 within the initial hours (2 h) of internalization by epithelial cells, followed by an increase of transcription at a later time (6 h) (11, 34). Although the findings (Fig. 5) might be interpreted as evidence that Tet38 removes chloroquine from the phagolysosome, this hypothesis is unlikely since if there is export of chloroquine by Tet38 in S. aureus cells, it would be transported from the bacterial cytoplasm into the phagolysosome. Furthermore, although CD36 is known to traffic in endosomes, internalization experiments argue that CD36 and Tet38 interact at the neutral pH in the uptake experiments and would not support a CD36-mediated model for endosomal release of S. aureus when the pH of the phagolysosome is raised. The role of Tet38 in extruding various substrates, including certain unsaturated fatty acids, as well as its location in the cell membrane suggests that Tet38 might also participate in maintaining the staphylococcal membrane fluidity and composition. This hypothesis would explain the perturbation that occurred in the absence of this transporter. Such a case was previously reported with the QacA efflux pump of S. aureus, where a qacA-bearing strain exhibited larger membrane fluidity than its parental strain while conferring resistance to a cationic antimicrobial polypeptide, tPMP-1 (35). Future work will be necessary to define other roles of Tet38 and the effects of pH on Tet38 function in the phagolysosome.
In summary, we have shown that interaction of Tet38 with CD36 contributes to the internalization of S. aureus into nonprofessional phagocytes such as A549 epithelial cells. In addition, Tet38 also affects trafficking to and from the phagolysosome, contributing to the ability of intracellular S. aureus to replicate and remain outside acidic, and strikingly outside nonacidic, phagolysosomes. These findings highlight the multiple functions of bacterial efflux pumps that extend beyond their ability to confer resistance to antimicrobials and host antibacterial substances to function in support of bacterial pathogenesis, thereby linking fitness and resistance capabilities.
MATERIALS AND METHODS
Cell lines, bacterial strains, and culture media.
The bacterial strains, plasmids, and cell lines used in this study are listed in Table 2. Human lung adenocarcinoma A549 cells and Jurkat cells were purchased from ATCC (CCL-185) and were cultivated in Dulbecco's modified Eagle's medium (DMEM) and RPMI medium (Life Technologies, Grand Island, NY), supplemented with 10% fetal bovine serum (FBS) and 4 mM l-glutamine (Fisher Scientific, Waltham, MA). The medium is referred to as assay medium depending on the cell lines used in the assays. For confocal microscopy experiments, DMEM without phenol red and pyruvate was used. A549 cells were grown at 37°C in 5% CO2. The S. aureus parental strain RN6390 was compared with the tet38 isogenic mutant QT7 (tet38::cat) to assess their ability to survive inside epithelial cells following standard invasion assays. The growth curves of RN6390 and QT7 were carried out using Luria-Bertani (LB) broth with the pH adjusted to 4.5 or 7. Chloroquine, ampicillin, gentamicin, Triton-X, and lysostaphin were purchased from Sigma (Sigma-Aldrich, St. Louis, MO).
TABLE 2.
Bacterial strains, plasmids, and cell lines used in this study
| Strain, plasmid, or cell line | Genotype or relevant characteristic(s) | Reference or source |
|---|---|---|
| S. aureus strains | ||
| RN6390 | 8325-4 wild type | 39 |
| QT7 | 8325-4 tet38::cat or Δtet38 | 40 |
| RN6390(pLZ113-tet38) | tet38 overexpressor | This study |
| RN6390(pAH16) | This study | |
| QT7(pAH16) | This study | |
| Plasmids | ||
| pAH14 | Plasmid for sarA P1-dependent YFP 10B expression, Ermr | 36 |
| pAH16 | pAH14 with sod ribosome binding site | 36 |
| pLZ113 | E. coli-S. aureus shuttle vector pRB373 with xyl/tet promoter cloned into the multiple cloning site | 41 |
| Cell lines | ||
| A549 | Human lung adenocarcinoma | 11 |
| Jurkat | Leukemic T-cell lymphoblast | 11 |
S. aureus expressing YFP protein from a plasmid.
Plasmid pAH16 carries the sarA P1 promoter and the ribosome-binding site of the sod gene that drive expression of the YFP 10B fluorescent reporter protein. This plasmid confers resistance to erythromycin (10 μg/ml) and provides fluorescence in S. aureus transformants (36). RN6390 and QT7 were transformed with plasmid pAH16 by electroporation and plated on LB agar plates supplemented with 10 μg/ml erythromycin to select YFP-expressing transformants.
S. aureus growth in the presence of acid pH and chloroquine (in vitro).
S. aureus RN6390 and QT7 were prepared from overnight cultures and grown until the optical density at 600 nm (OD600) reached 0.4. At this time point (time zero), two series of tubes (one series for RN6390 and one series for QT7) with 10 ml of bacterial culture in each tube were centrifuged to collect the bacterial pellets. Each pellet was resuspended into 10 ml of LB broth at pH 4.5 or at pH 7.0. To assess the effect of chloroquine on bacterial growth, we resuspended the bacterial pellet for each strain in 10 ml of LB broth at pH 7.0, supplemented with chloroquine at 20 μM. The cultures were then incubated under shaking at 37°C. Culture samples (1 ml) were taken hourly up to 6 h to measure the OD600 and for serial dilution and plating on LB agar plates.
Internalization of S. aureus by A549 epithelial cells.
The internalization assays were performed as previously described with some modifications (22). A549 cells were cultured in 5 ml of assay medium until they reached 90% confluence in a 25-ml tissue culture flask and were then seeded into 24-well plates (Costar) in assay medium and grown again to 90% confluence. The A549 cell concentration was adjusted to 104 cells/ml. S. aureus RN6390 or QT7 was prepared from overnight cultures and grown to an OD600 of 0.4. The bacteria were washed twice with 1× phosphate-buffered saline (PBS), and the bacterial pellet was resuspended in 10 ml of fresh assay medium to a concentration of 106 CFU/ml. For internalization assays and confocal microscopy imaging, A549 cells were infected with S. aureus at a multiplicity of infection (MOI) of 100 bacteria per epithelial cell (MOI of 100:1; 106 washed bacteria/104 A549 cells). The 24-well plates were centrifuged quickly for 1 min at 500 × g to allow bacterial adhesion to the cell monolayer. The bacterium-cell mixtures were incubated at 37°C in 5% CO2 for 2 h, and then the infected monolayers were washed three times with 1× PBS to remove residual nonadherent bacteria. The washed monolayers were incubated for 30 min at 37°C in 5% CO2 in assay medium with 200 μg/ml gentamicin and 20 μg/ml lysostaphin. Monolayers were again washed three times with 1× PBS, and the A549 epithelial cells were lysed with 200 μl of Triton X-100 (1×). Bacteria were diluted in PBS and plated on LB agar plates, and colony counts were performed to determine the number of viable intracellular bacteria. To assess the intracellular viability of S. aureus over time, the monolayers were incubated at 37°C in 5% CO2 in assay medium with 200 μg/ml gentamicin and 20 μg/ml lysostaphin for an additional 60, 120, and 180 min prior to plating. The tet38-overexpressor RN6390(pLZ113-tet38) and the complemented mutant strain QT7(pLZ113-tet38) were used as additional controls for the effect of tet38 on bacterial internalization.
Intracellular survival of S. aureus in the presence of chloroquine.
The intracellular survival assay was based on the method of Leimer et al. (25). Gentamicin- and lysostaphin-containing medium was supplemented with chloroquine to a final concentration of 20 μM (G/L medium-chloroquine) and used in the survival assays. Gentamicin and lysostaphin medium (G/L medium) was used as a control. A549 cells were infected with S. aureus RN6390 or QT7 as described above at an MOI of 100. Bacterial internalization was carried out for 2 h, and the monolayers were washed three times with 1× PBS and then treated with G/L medium for 2 h to eliminate extracellular bacteria. Absence of bacteria at the surface of the monolayers was verified by plating of the assay medium on LB agar plates. The monolayers were again washed three times with 1× PBS, and new medium (G/L medium-chloroquine or G/L medium) as a control was added to the monolayers. The assays were carried out over 6 days, with fresh medium added to the infected monolayers daily. At each time point, the monolayers were washed with 1× PBS and lysed with 150 μl of Triton-X. Serial dilutions of the cell lysates were performed, followed by plating on LB agar plates. Integrity of the A549 cells was verified by microscopy with or without invasion by S. aureus RN6390 or QT7.
Confocal microscopy of intracellular S. aureus.
A549 epithelial cells were cultivated in assay medium without phenol red (Fisher Scientific, Waltham, MA), seeded into an eight-chambered cover glass (250 μl per chamber), and incubated at 37°C and 5% CO2 overnight. Following addition of the dye FM4-64 (which stains cell membranes and organelles) according to the manufacturer's recommendation (50 μM final concentration; 20× dilution from the stock solution of 1 mM) or of the red fluorescent dye LysoTracker DND99 (1 μM final concentration; 1,000× dilution from the stock solution of 1 mM) (Fisher Scientific, Waltham, MA), the cells were incubated for 30 min at 37°C and 5% CO2 for the staining step. After staining, the A549 cells were washed twice with 1× PBS, and 250 μl of fresh medium without phenol red was added. RN6390 and QT7 transformed with the YFP fluorescent plasmid pAH16 were subcultured from an overnight culture and incubated until the OD600 reached 0.4. The bacterial cultures were adjusted to yield an MOI of 100 bacteria per A549 cell for the internalization assays. The assays were carried out as described above, and confocal imaging was done every hour following treatment of the infected monolayers with G/L medium up to 5 h. In assays performed with chloroquine-supplemented medium, the imaging was carried out for a period of 4 days to assess the survival of S. aureus after fusion of the bacterium-containing endosomes with lysosomes. A chambered cover glass was mounted onto a Nikon Ti-E inverted microscope fitted with a spinning disc confocal detection head (Yokogawa, Sugar Land, TX). A 4-W, continuous-wave laser (Coherent, Santa Clara, CA) or solid-state UV laser was used to produce excitation wavelengths of 405 nm or 647 nm. Cells and bacteria were imaged using a Nikon 100× objective with a high numerical aperture (NA) (1.49 NA, oil immersion objective; Nikon). Images were captured using an electron-multiplying charge-coupled device (EM-CCD) camera (C9100-13; Hamamatsu, Bridgewater, NJ) and analyzed using MetaMorph software (Molecular Devices, Downington, PA) (37).
Quantitation and localization of internalized bacteria.
The bacterial internalization assays (S. aureus with A549 cells) for confocal microscopy imaging were carried out as described above. A549 cells were stained with LysoTracker DND99. Following 30 min of incubation in G/L assay medium at 37°C under 5% CO2, the S. aureus-infected monolayers were washed three times with 1× PBS, and new G/L assay medium without phenol red was added to each chamber of the eight-chambered cover glass. The imaging was focused on various fields of S. aureus-internalized A549 cells, and an average of 10 images (10 fields) was captured for each S. aureus strain (RN6390 or QT7). The number of bacteria within six host cells in each of 10 successive fields was counted at each time point. The bacteria were represented as green dots (nonassociated with lysosomes) or yellow dots (associated with phagolysosomes). The bacterial counts are the average numbers taken from three separate experiments for each strain and condition. We counted the bacteria of 60 A549 cells in 10 confocal image fields. Each experiment was done in triplicate.
Internalization of S. aureus into A549 cells treated with anti-CD36, anti-Fn, and anti-TLR2 antibodies.
The standard assay described above was carried out with some modifications. Prior to adding S. aureus to the monolayers, the host cells were preincubated at 37°C under 5% CO2 for 30 min in assay medium containing 1% bovine serum albumin (BSA) plus anti-CD36, anti-Fn, or anti-TLR2 antibody (Life Technology, Grand Island, NY) at a final concentration of 50 nM. This assay was based on a prior study of the heat shock protein Hsp60 (13). The cell cultures, without washing, were infected with S. aureus RN6390 or QT7 according to the standard protocol. The number of intracellular bacteria was enumerated as above, and each assay was repeated three times. Internalization assays with or without added antibody were also carried out using Jurkat cells, a CD36-nonexpressing cell line, as a control (38). A mouse IgG nonspecific isotype control (Life Technology, Grand Island, NY) was used at the same concentration (50 nM) as the antibodies tested in the assays and served as a negative control for the tested antibodies.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants R37-AI23988 and P01-AI083214 (M. Gilmore, Principal Investigator) from the National Institutes of Health to D.C.H.
We are grateful to Alexander Horswill, who provided the plasmid pAH16 used in this study.
REFERENCES
- 1.Al-Wali WI, Elvin SJ, Mason CM, Clark A, Tranter HS. 1998. Comparative phenotypic characteristics of Staphylococcus aureus isolates from line and non-line associated septicaemia, CAPD peritonitis, bone/joint infections and healthy nasal carriers. J Med Microbiol 47:265–274. doi: 10.1099/00222615-47-3-265. [DOI] [PubMed] [Google Scholar]
- 2.Boyce JM, Cookson B, Christiansen K, Hori S, Vuopio-Varkila J, Kocagoz S, Oztop AY, Vandenbroucke-Grauls CM, Harbarth S, Pittet D. 2005. Methicillin-resistant Staphylococcus aureus. Lancet Infect Dis 5:653–663. doi: 10.1016/S1473-3099(05)70243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley SF. 2002. Staphylococcus aureus infections and antibiotic resistance in older adults. Clin Infect Dis 34:211–216. doi: 10.1086/338150. [DOI] [PubMed] [Google Scholar]
- 4.Drilling A, Coombs GW, Tan HL, Pearson JC, Boase S, Psaltis A, Speck P, Vreugde S, Wormald PJ. 2014. Cousins, siblings, or copies: the genomics of recurrent Staphylococcus aureus infections in chronic rhinosinusitis. Int Forum Allergy Rhinol 4:953–960. doi: 10.1002/alr.21423. [DOI] [PubMed] [Google Scholar]
- 5.Loughran AJ, Gaddy D, Beenken KE, Meeker DG, Morello R, Zhao H, Byrum SD, Tackett AJ, Cassat JE, Smeltzer MS. 2016. Impact of sarA and phenol-soluble modulins on the pathogenesis of osteomyelitis in diverse clinical isolates of Staphylococcus aureus. Infect Immun 84:2586–2594. doi: 10.1128/IAI.00152-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waters V. 2012. New treatments for emerging cystic fibrosis pathogens other than Pseudomonas. Curr Pharm Des 18:696–725. doi: 10.2174/138161212799315939. [DOI] [PubMed] [Google Scholar]
- 7.Abele-Horn M, Schupfner B, Emmerling P, Waldner H, Göring H. 2000. Persistent wound infection after herniotomy associated with small-colony variants of Staphylococcus aureus. Infection 28:53–54. doi: 10.1007/s150100050014. [DOI] [PubMed] [Google Scholar]
- 8.Aldridge KE, Gelfand MS, Schiro DD, Barg NL. 1992. The rapid emergence of fluoroquinolone-methicillin-resistant Staphylococcus aureus infections in a community hospital. An in vitro look at alternative antimicrobial agents. Diagn Microbiol Infect Dis 15:601–608. [DOI] [PubMed] [Google Scholar]
- 9.Alexander EH, Hudson MC. 2001. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl Microbiol Biotechnol 56:361–366. doi: 10.1007/s002530100703. [DOI] [PubMed] [Google Scholar]
- 10.Aubry-Damon H, Legrand P, Brun-Buisson C, Astier A, Soussy CJ, Leclercq R. 1997. Reemergence of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus: roles of an infection control program and changes in aminoglycoside use. Clin Infect Dis 25:647–653. doi: 10.1086/513749. [DOI] [PubMed] [Google Scholar]
- 11.Garzoni C, Francois P, Huyghe A, Couzinet S, Tapparel C, Charbonnier Y, Renzoni A, Lucchini S, Lew DP, Vaudaux P, Kelley WL, Schrenzel J. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8:171. doi: 10.1186/1471-2164-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923–932. [DOI] [PubMed] [Google Scholar]
- 13.Dziewanowska K, Carson AR, Patti JM, Deobald CF, Bayles KW, Bohach GA. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect Immun 68:6321–6328. doi: 10.1128/IAI.68.11.6321-6328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zapotoczna M, Jevnikar Z, Miajlovic H, Kos J, Foster TJ. 2013. Iron-regulated surface determinant B (IsdB) promotes Staphylococcus aureus adherence to and internalization by non-phagocytic human cells. Cell Microbiol 15:1026–1041. doi: 10.1111/cmi.12097. [DOI] [PubMed] [Google Scholar]
- 15.Nilsen NJ, Deininger S, Nonstad U, Skjeldal F, Husebye H, Rodionov D, von Aulock S, Hartung T, Lien E, Bakke O, Espevik T. 2008. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol 84:280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leelahavanichkul A, Bocharov AV, Kurlander R, Baranova IN, Vishnyakova TG, Souza AC, Hu X, Doi K, Vaisman B, Amar M, Sviridov D, Chen Z, Remaley AT, Csako G, Patterson AP, Yuen PS, Star RA, Eggerman TL. 2012. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol 188:2749–2758. doi: 10.4049/jimmunol.1003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, Patterson AP, Eggerman TL. 2008. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol 181:7147–7156. doi: 10.4049/jimmunol.181.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glatz JF, Nabben M, Heather LC, Bonen A, Luiken JJ. 2016. Regulation of the subcellular trafficking of CD36, a major determinant of cardiac fatty acid utilization. Biochim Biophys Acta 1860:1461–1471. doi: 10.1016/j.bbalip.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Silverstein RL, Febbraio M. 2009. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. 2005. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol 170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truong-Bolduc QC, Villet RA, Estabrooks ZA, Hooper DC. 2014. Native efflux pumps contribute resistance to antimicrobials of skin and the ability of Staphylococcus aureus to colonize skin. J Infect Dis 209:1485–1493. doi: 10.1093/infdis/jit660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong-Bolduc QC, Bolduc GR, Medeiros H, Vyas JM, Wang Y, Hooper DC. 2015. Role of the Tet38 efflux pump in Staphylococcus aureus internalization and survival in epithelial cells. Infect Immun 83:4362–4372. doi: 10.1128/IAI.00723-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahl BC, Goulian M, van Wamel W, Herrmann M, Simon SM, Kaplan G, Peters G, Cheung AL. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun 68:5385–5392. doi: 10.1128/IAI.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menzies BE, Kourteva I. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun 66:5994–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leimer N, Rachmuhl C, Palheiros MM, Bahlmann AS, Furrer A, Eichenseher F, Seidl K, Matt U, Loessner MJ, Schuepbach RA, Zinkernagel AS. 2016. Nonstable Staphylococcus aureus small-colony variants are induced by low pH and sensitized to antimicrobial therapy by phagolysosomal alkalinization. J Infect Dis 213:305–313. doi: 10.1093/infdis/jiv388. [DOI] [PubMed] [Google Scholar]
- 26.Dunmore BJ, Drake KM, Upton PD, Toshner MR, Aldred MA, Morrell NW. 2013. The lysosomal inhibitor, chloroquine, increases cell surface BMPR-II levels and restores BMP9 signalling in endothelial cells harbouring BMPR-II mutations. Hum Mol Genet 22:3667–3679. doi: 10.1093/hmg/ddt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alva-Murillo N, Lopez-Meza JE, Ochoa-Zarzosa A. 2014. Nonprofessional phagocytic cell receptors involved in Staphylococcus aureus internalization. Biomed Res Int 2014:538546. doi: 10.1155/2014/538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flock M, Flock JI. 2001. Rebinding of extracellular adherence protein Eap to Staphylococcus aureus can occur through a surface-bound neutral phosphatase. J Bacteriol 183:3999–4003. doi: 10.1128/JB.183.13.3999-4003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster TJ, Höök M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 6:484–488. doi: 10.1016/S0966-842X(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 30.Peacock SJ, Foster TJ, Cameron BJ, Berendt AR. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 31.Simpson KH, Bowden G, Höök M, Anvari B. 2003. Measurement of adhesive forces between individual Staphylococcus aureus MSCRAMMs and protein-coated surfaces by use of optical tweezers. J Bacteriol 185:2031–2035. doi: 10.1128/JB.185.6.2031-2035.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. 2005. CD36 is a sensor of diacylglycerides. Nature 433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 33.Lukacs GL, Rotstein OD, Grinstein S. 1990. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem 265:21099–21107. [PubMed] [Google Scholar]
- 34.Anderson KL, Roux CM, Olson MW, Luong TT, Lee CY, Olson R, Dunman PM. 2010. Characterizing the effects of inorganic acid and alkaline shock on the Staphylococcus aureus transcriptome and messenger RNA turnover. FEMS Immunol Med Microbiol 60:208–250. doi: 10.1111/j.1574-695X.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayer AS, Kupferwasser LI, Brown MH, Skurray RA, Grkovic S, Jones T, Mukhopadhay K, Yeaman MR. 2006. Low-level resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein 1 in vitro associated with qacA gene carriage is independent of multidrug efflux pump activity. Antimicrob Agents Chemother 50:2448–2454. doi: 10.1128/AAC.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR. 2009. Fluorescent reporters for Staphylococcus aureus. J Microbiol Methods 77:251–260. doi: 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tam JM, Mansour MK, Khan NS, Yoder NC, Vyas JM. 2012. Use of fungal derived polysaccharide-conjugated particles to probe dectin-1 responses in innate immunity. Integr Biol (Camb.) 4:220–227. doi: 10.1039/C2IB00089J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armesilla AL, Calvo D, Vega MA. 1996. Structural and functional characterization of the human CD36 gene promoter: identification of a proximal PEBP2/CBF site. J Biol Chem 271:7781–7787. doi: 10.1074/jbc.271.13.7781. [DOI] [PubMed] [Google Scholar]
- 39.Truong-Bolduc QC, Liao C-H, Villet R, Bolduc GR, Estabrooks Z, Taguezem GF, Hooper DC. 2012. Reduced aeration affects the expression of the NorB efflux pump of Staphylococcus aureus by posttranslational modification of MgrA. J Bacteriol 194:1823–1834. doi: 10.1128/JB.06503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. 2005. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J Bacteriol 187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Fan F, Palmer LM, Lonetto MA, Petit C, Voelker LL, St John A, Bankosky B, Rosenberg M, McDevitt D. 2000. Regulated gene expression in Staphylococcus aureus for identifying conditional lethal phenotypes and antibiotic mode of action. Gene 255:297–305. doi: 10.1016/S0378-1119(00)00325-5. [DOI] [PubMed] [Google Scholar]