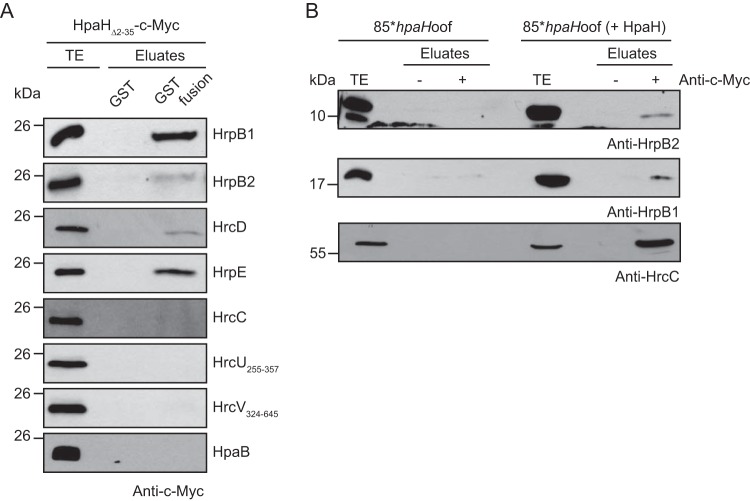
FIG 3.
HpaH interacts with periplasmic components of the T3S system. (A) In vitro GST pulldown assays with HpaHΔ2–35–c-Myc. GST and GST fusions of HrpB1, HrpB2, HrcD, HrpE, HrcC, HrcU255–357, HrcV324–645, and HpaB as indicated were immobilized on glutathione-Sepharose and incubated with a bacterial lysate containing HpaHΔ2–35–c-Myc. Total cell extracts (TE) and eluates were analyzed by immunoblotting using a c-Myc epitope-specific antibody. All GST fusion proteins were stably synthesized, as shown in Fig. S4 in the supplemental material. It was previously shown that HrpB1, HrpB2, HrcD, and HrpE do not unspecifically interact with T3S-associated proteins (24, 35). (B) Coimmunoprecipitation experiments with X. campestris pv. vesicatoria. Strain 85*hpaHoof with or without HpaH–c-Myc was grown in secretion medium. Bacterial lysates were incubated in the presence (+) or absence (−) of c-Myc epitope-specific antibodies coupled to protein G-Sepharose. Bacterial total cell extracts (TE) and precipitated proteins (eluate) were analyzed by immunoblotting using antibodies specific for HrpB1, HrpB2, and HrcC.