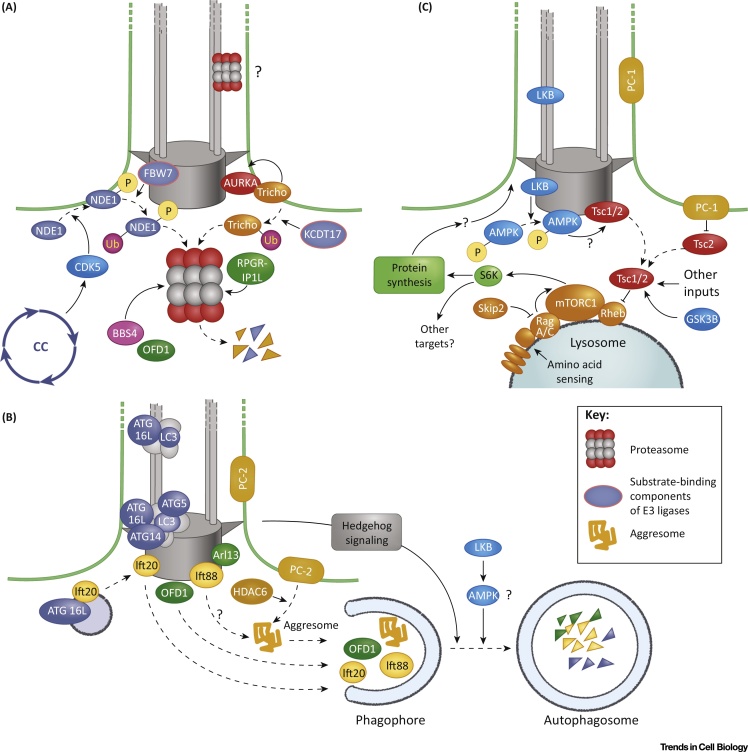
Figure 3.
Relationships Between Cellular Proteostasis and Ciliogenesis. (A) Selected UPS-driven regulatory processes in ciliogenesis. At exit from the cell cycle (CC), NDE1 is phosphorylated, which primes it for ubiquitination (‘Ub’ in purple circle). Similarly, trichoplein (Tricho) is ubiquitinated and degraded following cell-cycle exit. RPGRIP1L (also known as MKS5) is a ciliary transition zone protein that directly binds to a regulatory component of the 19S proteasome subunit. (B) Current understanding of functional relationships between the TOR pathway and ciliogenesis. TOR pathway upregulation by the suppression of TSC1/2 or Skip2 function leads to cilia elongation. In a reciprocal mechanism, cilia cause TOR downregulation through LKB and presumably AMPK kinases in response to mechanical stimuli. Although AMPK is known to upregulate TSC2 activity in response to a low-energy state, it is not clear whether it regulates TSC2 in the context of ciliogenesis. TSC1 does localize to the cilia base, and in several experimental contexts its loss-of-function results in cilia elongation. The significance of its ciliary localization is, however, not clear because it functions by activating lysosome membrane-bound Rheb. Loss of cilia as well as polycystin-1 (PC-1) mutations upregulate TOR. Although polycystin-1 upregulates TSC2 by influencing its phosphorylation state, this mechanism may not involve cilia. (C) The current understanding of reciprocal functional relationships between ciliogenesis and autophagy. Following serum withdrawal, autophagy promotes ciliogenesis by eliminating OFD1. By contrast, during sustained serum starvation autophagy appears to downregulate ciliogenesis by eliminating IFT20. Ciliary proteins, such as IFT20 and polycystin-2, enhance autophagy, perhaps by stimulating transport of ATG protein(s) to cilia. Cilia also stimulate autophagy through the Hedgehog pathway. Finally, HDAC6-mediated aggregasome formation may mediate the elimination of damaged ciliary proteins.