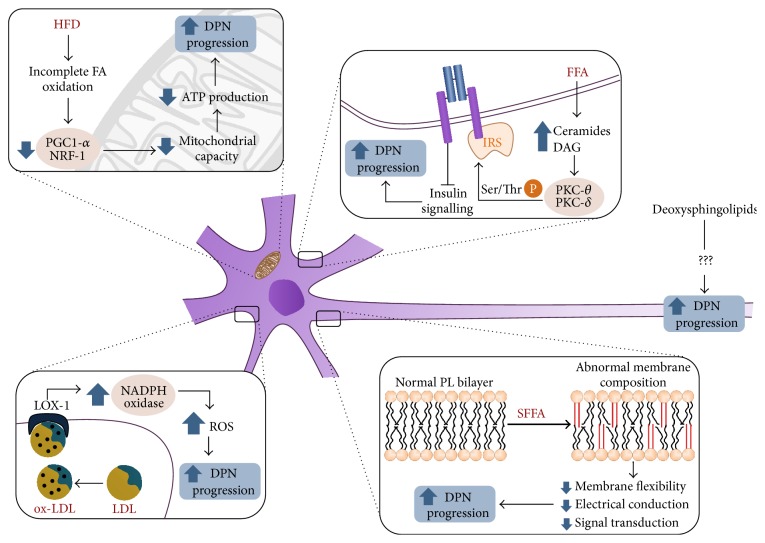
Figure 1.
Pathogenic mechanisms linking abnormal lipid metabolism to progression of diabetic neuropathy. HFD: high-fat diet, FA: fatty acids, PGC-1alpha: PPAR-gamma coactivator 1-alpha, NRF-1: nuclear respiratory factor-1, DPN: diabetic polyneuropathy, FFA: free fatty acids, IRS: insulin receptor substrates, PKC-theta: protein kinase C, theta isoform, PKC-delta: protein kinase C, delta isoform, Ser/ThrP: phosphorylation in serine or threonine, ox-LDL: oxidized LDL, LOX-1: lectin-like oxidized LDL receptor, NADPH oxidase: reduced nicotinamide-adenine dinucleotide phosphate oxidase, PL: phospholipid, and SFFA: saturated free fatty acids. Insulin resistance or a high-fat diet increase the cellular supply of FFA, leading to decreased expression of PGC-1alpha and NRF1-alpha-responsive genes and subsequently to impaired mitochondrial capacity and nerve dysfunction. Increased supply of FFA also causes uncontrolled formation of DAG and ceramides, which activate atypical PKC isoforms and promote serine/threonine phosphorylation of IRS, decreased insulin signaling, and defective nerve growth and repair. The augmented availability of SFFA in insulin resistance leads to changes in the fatty acid composition of plasma membrane phospholipids. Membranes richer in saturated FA are more rigid and exhibit disturbances of electrical conduction and a reduced capacity for receptor expression and signal transduction, all of which worsen DPN. Accelerated ROS production in diabetes generates oxLDL that bind to the LOX-1 receptor and activate NADPH oxidase, worsening ROS production even further and hastening the progression of DPN. Finally, oxidized deoxysphingolipids are neurotoxic lipids associated with DPN, but their mechanism of action is still unknown.