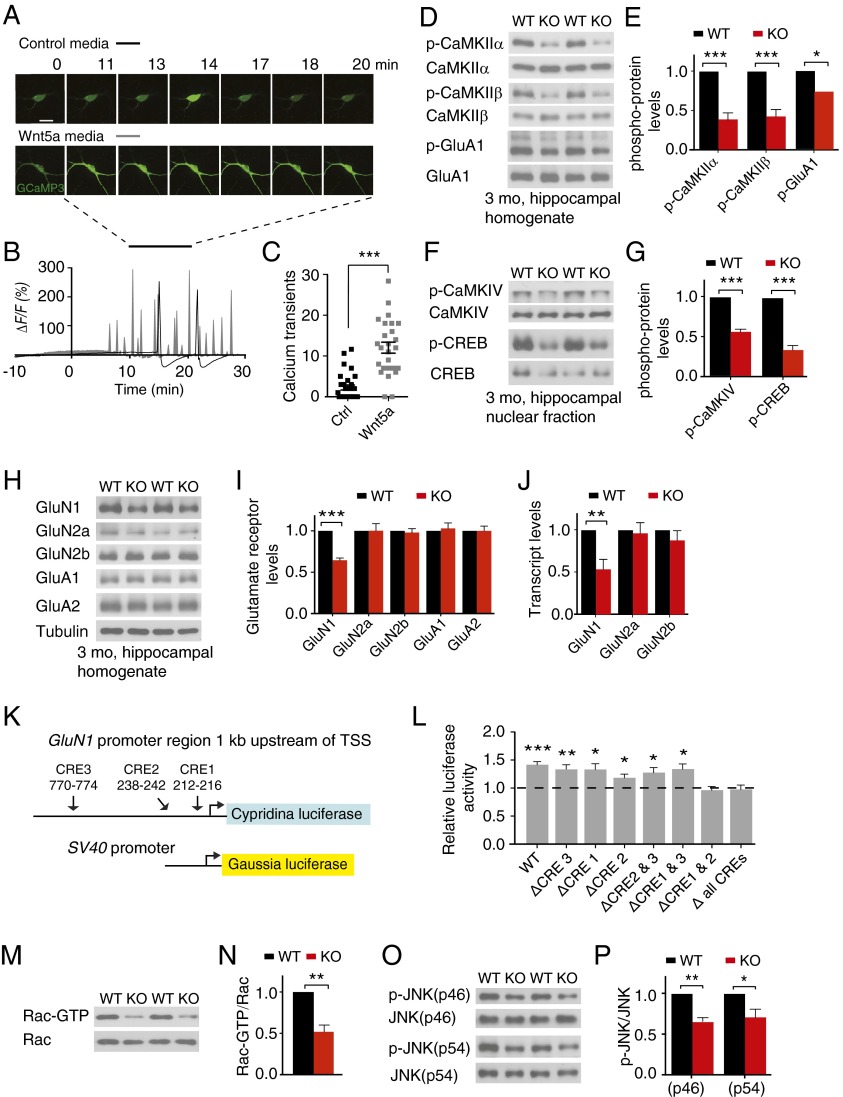
Fig. 4.
Wnt5a loss disrupts calcium and cytoskeletal signaling and decreases CREB-mediated GluN1 synthesis. (A) Wnt5a elicits a robust increase in calcium transients in hippocampal neurons. Rat hippocampal neuron cultures were transfected with GCaMP3 and treated with control L- or Wnt5a-conditioned media for 30 min. (Scale bar, 20 μm.) (B) Representative traces of calcium transients. (C) Wnt5a elicits a fivefold increase in calcium transients compared with control media. Results are mean ± SEM from n = 3 independent experiments (total of n = 31 L media-treated cells and n = 26 Wnt5a-treated neurons); ***P < 0.001, two-tailed t test. (D and E) Phosphorylated CaMKIIα/β and phosphorylation of GluA1Ser831 are significantly reduced in CaMKII-Wnt5afl/fl (KO) hippocampus compared with Wnt5afl/fl litter-mates (WT) at 3 mo. Immunoblots were stripped and reprobed for total CaMKIIα, -β, and GluA1 for normalization. Results are mean ± SEM from n = 6 mice per genotype; *P < 0.05, ***P < 0.001, two-tailed t test. (F and G) CaMKIV and CREB phosphorylation are attenuated in hippocampal nuclear fractions from 3-mo-old KO mice. Blots were reprobed for total CaMKIV and CREB. Results are mean ± SEM from n = 6 and n = 5 mice per genotype; ***P < 0.001, two-tailed t test. (H and I) GluN1, the obligatory NMDA receptor subunit, is significantly decreased in 3-mo KO hippocampus, but levels of other NMDA receptor subunits, GluN2a/2b, and AMPA-type glutamate receptor subunits, GluA1 and GluA2, are unaltered. Immunoblots were stripped and reprobed for tubulin. Results are mean ± SEM from n = 6 mice per genotype; ***P < 0.001, two-tailed t test. (J) qPCR analysis shows decrease in GluN1 but not GluN2a/b transcripts in the KO hippocampus. Results are mean ± SEM from n = 6 mice per genotype; **P < 0.01, two-tailed t test. (K) A 1-kb region upstream of the mouse GluN1 promoter harbors three putative CRE sites. Hippocampal neurons were cotransfected with luciferase reporter constructs, Cypridina luciferase driven by the GluN1 promoter region, and Gaussia luciferase driven by an SV40 promoter as a control. Neurons were treated with control or Wnt5a media for 6 h and then harvested to measure luciferase activity. (L) Wnt5a treatment significantly increases GluN1 promoter-driven luciferase activity. Deletion of all three CRE sites or just the two proximal binding sites (CRE1 and CRE2) alone abolishes the Wnt5a-mediated response. Results are mean ± SEM for n = 6 independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001, two-tailed t test. (M and N) Rac1-GTP levels are reduced in 3-mo KO mice. Hippocampal homogenates were subjected to GST-PAK-PBD-agarose pull-downs for active Rac-GTP and immunoblotted for Rac1. Rac1-GTP signal intensities were normalized to total Rac1. Results are mean ± SEM from n = 6 mice per genotype; **P < 0.01, two-tailed t test. (O and P) Phospho-JNK levels are significantly decreased in hippocampal homogenates from 3-mo KO mice. Results are means ± SEM from n = 6 mice per genotype; *P < 0.05, **P < 0.01, two-tailed t test.