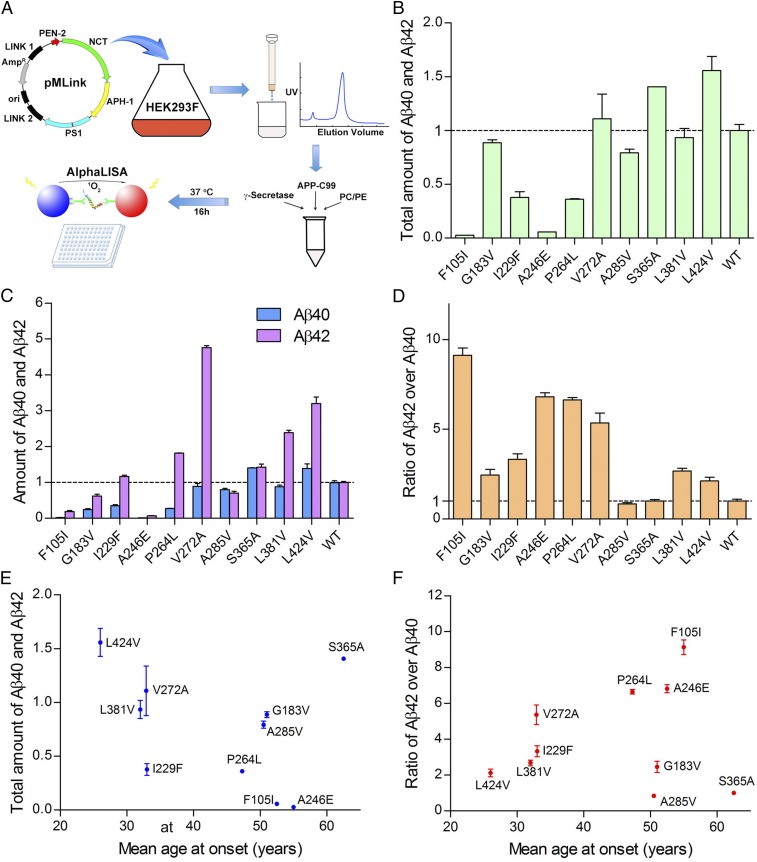
Fig. 1.
Preliminary analysis of 10 AD-derived PS1 mutations. (A) A schematic diagram of the major workflow in this study. The WT human γ-secretase and 138 variants, each containing an AD-derived mutation in PS1, were individually overexpressed and purified. The peak fractions were incubated with APP-C99 and examined for the production of Aβ42 and Aβ40 using the AlphaLISA assay. (B) Effect of 10 AD-derived PS1 mutations on the cleavage activities of the corresponding γ-secretases. Shown here is the combined production of Aβ42 and Aβ40 peptides by the 10 γ-secretase variants. The activity of WT γ-secretase was normalized as 1. Each experiment was repeated three times, and the SD is shown. (C) Effect of 10 AD-derived PS1 mutations on the generation of Aβ42 and Aβ40. (D) All but two variants show increased molar ratios of Aβ42 over Aβ40 compared with WT γ-secretase. Only the variant A285V displays a slightly lower Aβ42/Aβ40 ratio, and S365A is nearly identical to WT γ-secretase. (E) The combined production of Aβ42 and Aβ40 by each of the 10 γ-secretase variants shows no obvious correlation with the mean AAO. (F) The Aβ42/Aβ40 ratio exhibits no obvious correlation with the mean AAO.