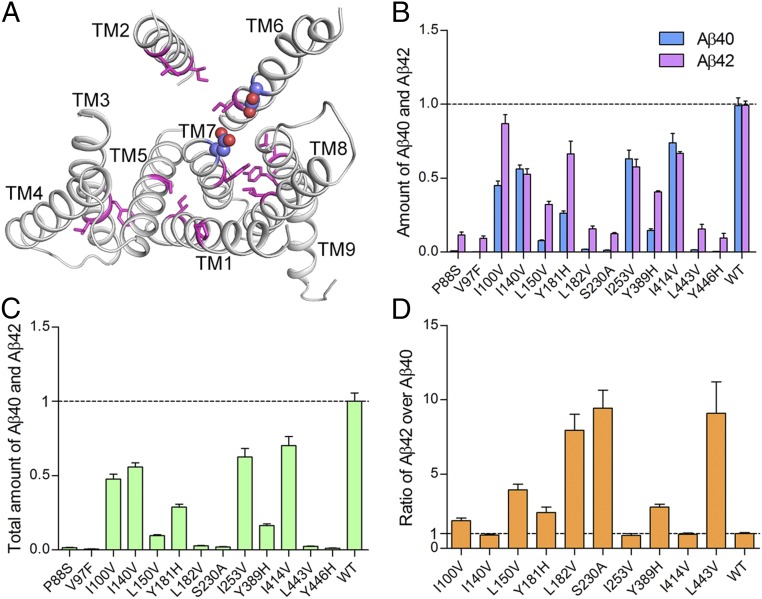
Fig. 6.
γ-secretase variants with engineered PS1 mutations exhibit similar biochemical properties as the variants with AD-derived mutations. (A) Location of the 13 amino acids affected by engineered mutations. Except TM4, each of the other eight TMs contributes at least one residue for mutation. Shown here is a ribbon representation of PS1 structure. The residues targeted for mutation are colored magenta, and the two catalytic Asp residues are shown in spheres. (B) All 13 engineered mutations result in compromised protease activities of the corresponding γ-secretase variants for the generation of both Aβ42 and Aβ40. (C) All 13 engineered mutations lead to decreased levels of protease activity as judged by the combined production of Aβ42 and Aβ40. (D) Seven mutations cause increased ratios of Aβ42/Aβ40 compared with WT. Only three mutations (I140V, I253V, and I414V) have no significant effect on the Aβ42/Aβ40 ratios.