Abstract
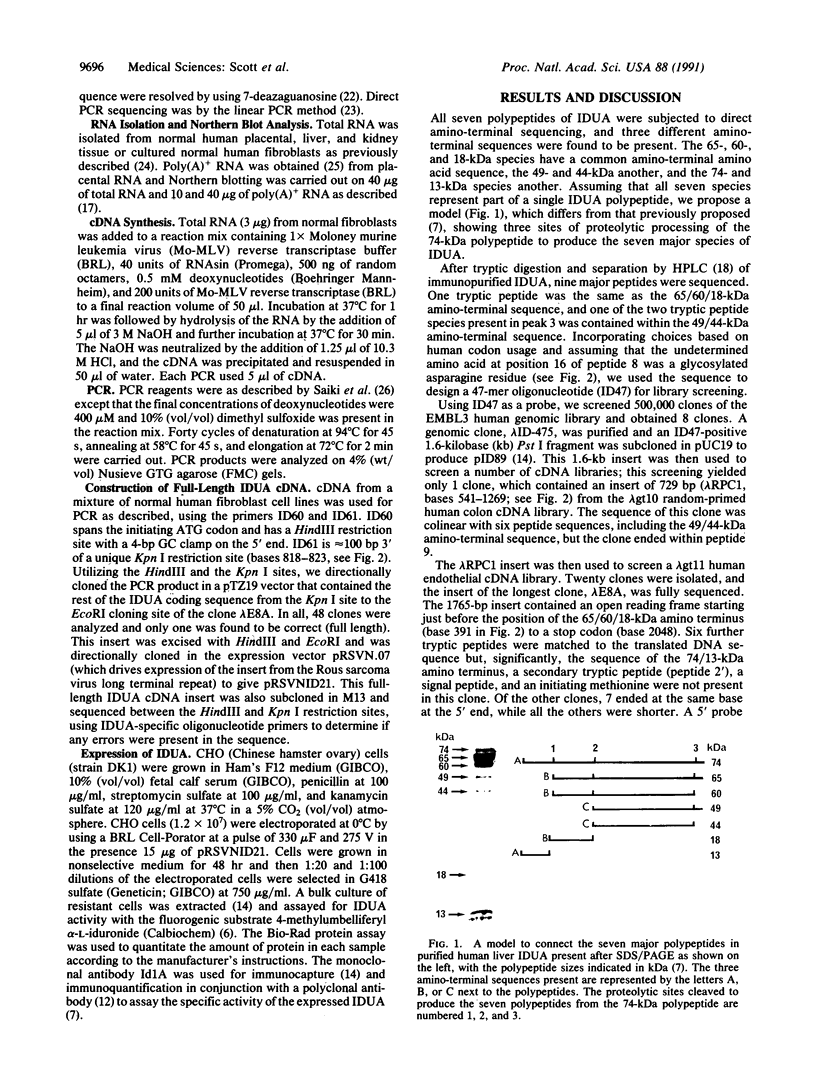
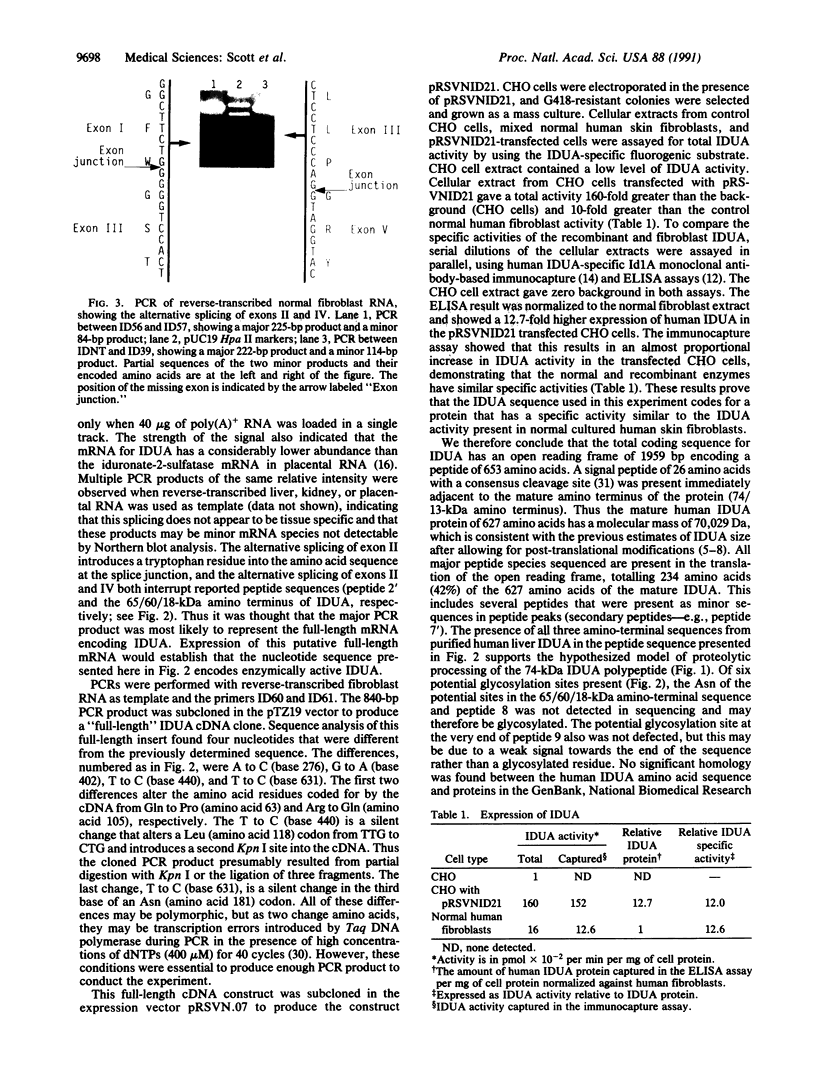
alpha-L-Iduronidase (IDUA; EC 3.2.1.76) is a lysosomal hydrolase in the metabolic pathway responsible for the degradation of the glycosaminoglycans heparan sulfate and dermatan sulfate. A deficiency of IDUA in humans leads to the accumulation of these glycosaminoglycans and results in the lysosomal storage disorder mucopolysaccharidosis type I. We have isolated and sequenced cDNA clones containing part of the human IDUA coding region and used PCR from reverse-transcribed RNA to obtain the full IDUA sequence. Analysis of the predicted 653-amino acid precursor protein shows that IDUA has a 26-amino acid signal peptide that is cleaved immediately prior to the amino terminus of the 74-kDa polypeptide present in human liver IDUA. The protein sequence contains six potential N-glycosylation sites. Northern blot analysis with IDUA cDNA detected only a single 2.3-kilobase mRNA species in human placental RNA; however, PCR analysis of fibroblast, liver, kidney, and placental RNA showed the existence of alternatively spliced mRNA from the IDUA gene. Southern blot analysis failed to detect major deletions or gene rearrangements in any of the 40 mucopolysaccharidosis type I patients studied. Expression of a full-length IDUA cDNA construct in Chinese hamster ovary cells produced human IDUA protein at a level 13-fold higher than, and with a specific activity comparable to, IDUA present in normal human fibroblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., McCourt P. A., Hopwood J. J. Immunopurification and characterization of human alpha-L-iduronidase with the use of monoclonal antibodies. Biochem J. 1989 Apr 1;259(1):199–208. doi: 10.1042/bj2590199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., Saccone G. T., Hopwood J. J. Human alpha-L-iduronidase. 1. Purification, monoclonal antibody production, native and subunit molecular mass. Eur J Biochem. 1985 Oct 1;152(1):21–28. doi: 10.1111/j.1432-1033.1985.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Eckert K. A., Kunkel T. A. High fidelity DNA synthesis by the Thermus aquaticus DNA polymerase. Nucleic Acids Res. 1990 Jul 11;18(13):3739–3744. doi: 10.1093/nar/18.13.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Morris C. P. The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med. 1990 Oct;7(5):381–404. [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. Biochemical discrimination of Hurler and Scheie syndromes. Clin Sci (Lond) 1979 Sep;57(3):265–272. doi: 10.1042/cs0570265. [DOI] [PubMed] [Google Scholar]
- MacDonald M. E., Scott H. S., Whaley W. L., Pohl T., Wasmuth J. J., Lehrach H., Morris C. P., Frischauf A. M., Hopwood J. J., Gusella J. F. Huntington disease-linked locus D4S111 exposed as the alpha-L-iduronidase gene. Somat Cell Mol Genet. 1991 Jul;17(4):421–425. doi: 10.1007/BF01233067. [DOI] [PubMed] [Google Scholar]
- Minami R., Fujibayashi S., Igarashi C., Ishikawa Y., Wagatsuma K., Nakao T., Tsugawa S. Heparan sulfate and dermatan sulfate from the liver of a patient with Hurler syndrome: high performance liquid chromatography of their degradation products after incubation with alpha-L-iduronidase-deficient fibroblasts. Clin Chim Acta. 1984 Feb 28;137(2):179–187. doi: 10.1016/0009-8981(84)90178-5. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreau H., Galjart N. J., Gillemans N., Willemsen R., van der Horst G. T., d'Azzo A. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem. 1989 Dec 5;264(34):20655–20663. [PubMed] [Google Scholar]
- Muller V. J., Hopwood J. J. alpha-L-Iduronidase deficiency in mucopolysaccharidosis type I against a radiolabelled sulfated disaccharide substrate derived from dermatan sulfate. Clin Genet. 1984 Nov;26(5):414–421. doi: 10.1111/j.1399-0004.1984.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R., Neufeld E. F. Maturation of alpha-L-iduronidase in cultured human fibroblasts. J Biol Chem. 1981 Mar 25;256(6):3044–3048. [PubMed] [Google Scholar]
- Oshima A., Kyle J. W., Miller R. D., Hoffmann J. W., Powell P. P., Grubb J. H., Sly W. S., Tropak M., Guise K. S., Gravel R. A. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci U S A. 1987 Feb;84(3):685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi K., Tsuchiyama A., Itakura Y., Sogawa H., Wagatsuma K., Nakao T., Sakamoto S., Yachi A. The mechanism of hyperammonaemia and hyperornithinaemia in the syndrome of hyperornithinaemia, hyperammonaemia with homocitrullinuria. J Inherit Metab Dis. 1983;6(3):133–134. doi: 10.1007/BF01800748. [DOI] [PubMed] [Google Scholar]
- Quintern L. E., Schuchman E. H., Levran O., Suchi M., Ferlinz K., Reinke H., Sandhoff K., Desnick R. J. Isolation of cDNA clones encoding human acid sphingomyelinase: occurrence of alternatively processed transcripts. EMBO J. 1989 Sep;8(9):2469–2473. doi: 10.1002/j.1460-2075.1989.tb08382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. A., Freeman C., Nelson P. V., Morris C. P., Hopwood J. J. Human glucosamine-6-sulfatase cDNA reveals homology with steroid sulfatase. Biochem Biophys Res Commun. 1988 Nov 30;157(1):218–224. doi: 10.1016/s0006-291x(88)80035-4. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. S., Ashton L. J., Eyre H. J., Baker E., Brooks D. A., Callen D. F., Sutherland G. R., Morris C. P., Hopwood J. J. Chromosomal localization of the human alpha-L-iduronidase gene (IDUA) to 4p16.3. Am J Hum Genet. 1990 Nov;47(5):802–807. [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. J., Hall C. W., Leder I. G., Neufeld E. F. The relationship of alpha-L-iduronidase and Hurler corrective factor. Arch Biochem Biophys. 1976 Jan;172(1):156–161. doi: 10.1016/0003-9861(76)90061-8. [DOI] [PubMed] [Google Scholar]
- Spellacy E., Shull R. M., Constantopoulos G., Neufeld E. F. A canine model of human alpha-L-iduronidase deficiency. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6091–6095. doi: 10.1073/pnas.80.19.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. A., Gibson G. J., Brooks D. A., Hopwood J. J. alpha-L-iduronidase in normal and mucopolysaccharidosis-type-I human skin fibroblasts. Biochem J. 1991 Feb 15;274(Pt 1):263–268. doi: 10.1042/bj2740263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. J., Morris C. P., Anson D. S., Occhiodoro T., Bielicki J., Clements P. R., Hopwood J. J. Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8531–8535. doi: 10.1073/pnas.87.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]




