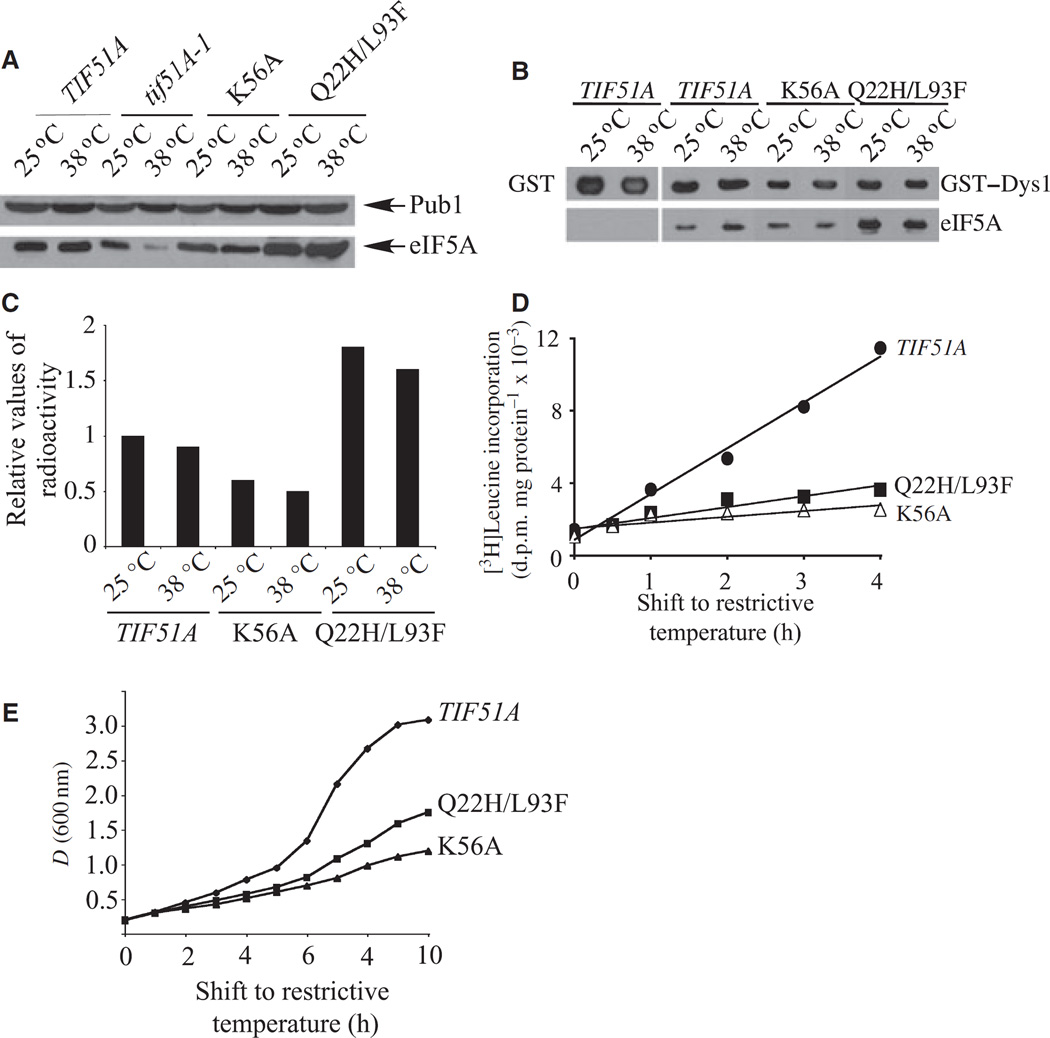
Fig. 3.
Characterization of two new elF5A mutants producing stable elF5A proteins. (A) Determination of mutant elF5A levels. Protein levels were analyzed as in Fig. 2C. (B) GST pulldown of wild-type and mutant elF5A with GST–Dys1 at permissive (25 °C) and nonpermissive (38 °C) temperatures. GST alone was used as a negative control (first pair of lanes). (C) Analysis of elF5A mutant proteins as substrates for hypusine formation. Yeast strains were cultured with [3H]spermidine and analyzed as described in Experimental procedures. The relative values of radioactivity incorporated into protein as deoxyhypusine/hypusine are shown. (D) The effects of wild-type and mutant elF5A on protein synthesis. Protein synthesis was measured as described in Experimental procedures and the degree of [3H]leucine incorporation into protein is shown as disintegrations per minute (d.p.m.)·µg−1 of protein. (E) Growth curves of wild-type and elF5A mutant strains. The strains were grown at 30 °C in rich medium (YPD) to mid-log phase. The cultures were then shifted to 38 °C and growth was monitored for 10 h by spectrophotometry (at 600 nm).