Figure 3. ΔSS mutant form of IL‐2Rα as a model for mislocalized proteins.
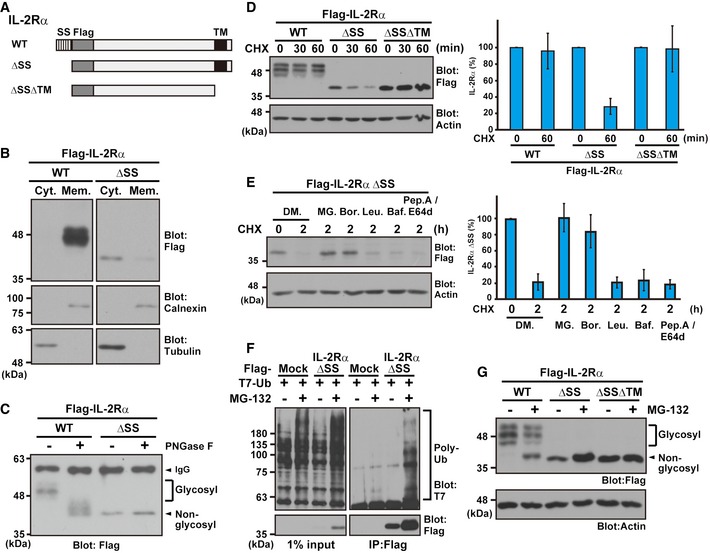
- Schematic representation of the IL‐2Rα proteins used in this study. WT: wild type, ΔSS: signal sequence (SS)‐deleted mutant, and ΔSSΔTM: SS and transmembrane domain (TM)‐deleted mutant.
- IL‐2Rα ΔSS mislocalized to the cytosolic fraction. HeLa cells, expressing Flag‐tagged IL‐2Rα WT or ΔSS, were fractionated to the cytosolic (Cyt.) and membrane (Mem.) fractions. Tubulin was used as a cytoplasmic marker, while calnexin was used for the ER membrane fraction.
- IL‐2Rα ΔSS was not glycosylated. Flag‐tagged IL‐2Rα WT or ΔSS proteins were expressed in HeLa cells and immunoprecipitated with an anti‐Flag antibody. The precipitates were incubated with (+) or without (−) 10 units of the deglycosylation enzyme PNGase F for 2 h and subjected to Western blot analysis with an anti‐Flag antibody. Glycosylated and non‐glycosylated signals are indicated.
- The glycosylated form of IL‐2Rα WT is a stable protein, while mislocalized IL‐2Rα ΔSS is degraded rapidly via its TMD. A series of IL‐2Rα deletion proteins were expressed in HeLa cells and then chased with 20 μg/ml CHX for the indicated periods. Actin was used as a loading control. The right panel is a quantification graph of the left blot signals, and data represent mean ± SD calculated from four independent experiments (n = 4).
- IL‐2Rα ΔSS is degraded in a proteasome‐dependent manner. HeLa cells expressing Flag‐tagged IL‐2Rα ΔSS were treated with 20 μg/ml CHX as well as 0.1% DMSO (DM.), 10 μM MG‐132 (MG.), 2 μM bortezomib (Bor.), 10 μM leupeptin (Leu.), 0.1 μM bafilomycin A1 (Baf.), or 10 μg/ml pepstatin A (pep. A)/E64d for the indicated periods. The right panel indicates the quantified data of the left blot signals presented as mean ± SD calculated from three independent experiments (n = 3).
- The Flag‐tagged IL‐2Rα ΔSS mutant and T7‐tagged ubiquitin were co‐expressed in HeLa cells, and the cells were treated with (+) or without (−) 10 μM MG‐132. After 4 h, Flag precipitates were blotted with anti‐T7 and anti‐Flag antibodies.
- HeLa cells expressing Flag‐tagged IL‐2Rα WT, ΔSS, and ΔSSΔTM proteins were treated with (+) or without (−) 10 μM MG‐132. At 4 h after MG‐132 treatment, the cells were lysed and analyzed by immunoblotting using the indicated antibodies. Note that a higher migrating form of non‐glycosylated (and thus defective) IL‐2Rα WT (indicated by the arrowhead) is detected strongly in the presence of MG‐132.
Source data are available online for this figure.
