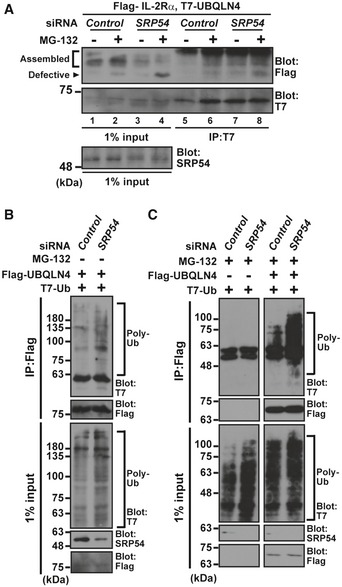
Figure 8. SRP deficiency enhances the interaction between UBQLN4 and its client proteins.
-
AUBQLN4 interacts with non‐glycosylated (and thus defective) IL‐2Rα WT in SRP54 knockdown cells. After treatment of HeLa cells with SRP54 siRNA (10 nM) for 48 h, Flag‐tagged IL‐2Rα WT and T7‐tagged UBQLN4 proteins were expressed, and the cells were treated with (+) or without (−) 10 μM MG‐132 at 4 h before harvesting. UBQLN4 was affinity‐purified from cell extracts and probed with an anti‐Flag antibody. Note that the levels of non‐glycosylated IL‐2Rα WT (indicated as defective) increased, while the glycosylated form of this protein (indicated as assembled) decreased in SRP54 knockdown cells.
-
B, CSRP54 knockdown stimulates polyubiquitinated proteins co‐precipitation of UBQLN4. Flag‐tagged UBQLN4 and T7‐tagged ubiquitin were expressed in SRP54 siRNA‐treated cells, and Flag precipitates were probed with an anti‐T7 antibody to detect polyubiquitinated client co‐precipitation with UBQLN4 under no addition of MG‐132 (B). Note that the interaction of UBQLN4 with polyubiquitinated substrates was further increased in SRP54 knockdown cells treated with MG‐132 for 4 h before harvesting (C).
Source data are available online for this figure.
