ABSTRACT
Previously, we showed that mutations in Mycobacterium tuberculosis panD, involved in coenzyme A biosynthesis, cause resistance against pyrazinoic acid, the bioactive component of the prodrug pyrazinamide. To identify additional resistance mechanisms, we isolated mutants resistant against pyrazinoic acid and subjected panD wild-type strains to whole-genome sequencing. Eight of the nine resistant strains harbored missense mutations in the unfoldase ClpC1 associated with the caseinolytic protease complex.
KEYWORDS: ClpC1, Mycobacterium tuberculosis, caseinolytic protease, pyrazinamide, resistance
TEXT
Pyrazinamide (PZA) is a critical component of the current first-line drug regimen to treat tuberculosis (TB). Inclusion of PZA in the regimen in the 1980s shortened the duration of therapy from 12 to 6 months (1). However, a 6-month regimen is still too lengthy for ensuring compliance, not only affecting cure rates, but also facilitating the development of drug resistance. Thus, shortening the treatment to 2 months or less is a major goal in TB drug development (2). Most new drug combinations under development include PZA, although its target(s) remains ill defined (3). Due to the clinically proven sterilizing activity of PZA, identifying its mechanism of action may provide clues to develop novel approaches for discovering shorter chemotherapeutic regimens.
PZA is a prodrug that must be converted to its bioactive form, pyrazinoic acid (POA). Prodrug conversion is carried out by the host's metabolism (4) and the bacterial amidase PncA, the inactivation of which causes PZA resistance in vitro (5). POA appears to have multiple bacterial targets. POA was proposed to act as an ionophore, causing intracellular acidification (6, 7), though this model was questioned (8). Biochemical and protein binding studies have identified at least two possible targets for POA, namely, fatty acid synthetase I (FASI) (9) and 30S ribosomal S1 protein (RpsA) (10). This suggests that POA may interfere with fatty acid synthesis and with trans-translation, which is a rescue mechanism that frees ribosomes stalled in translation. Recently, we demonstrated that at least two independent mechanisms of resistance to POA/PZA exist in Mycobacterium bovis BCG. First, high-level POA resistance is caused by missense mutations in aspartate decarboxylase panD (also reported in references 11 and 12), indicating that POA interferes with pantothenate and coenzyme A biosynthesis (13). Second, low-level POA resistance is caused by the loss of phthiocerol dimycocerosate (PDIM) virulence factor biosynthesis via frameshift mutations in the polyketide synthase genes mas and ppsA through ppsE (ppsA-E) (14). We also showed that the two resistance mechanisms were recapitulated in virulent Mycobacterium tuberculosis by whole-genome sequencing of 10 in vitro-isolated POA-resistant strains (14).
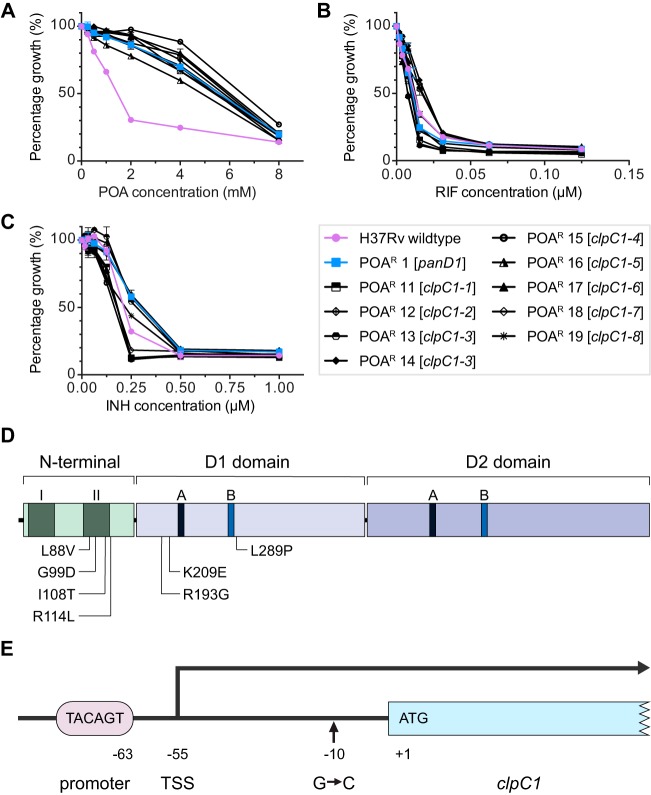
Here, we asked whether additional “panD-like” mechanisms, i.e., high-level POA/PZA resistance mechanisms independent of panD mutations, can be identified in M. tuberculosis. To avoid selecting strains with loss-of-function mutations in the prodrug-activating amidase PncA, we selected M. tuberculosis H37Rv directly on 7H10 agar containing POA, i.e., on agar containing the bioactive form of PZA instead of the prodrug. We carried out spontaneous mutant selection, colony purification on respective POA-containing agar to verify drug resistance, and cryopreservation of resistant strains for four independent batches of M. tuberculosis cultures by plating on 2 mM or 4 mM POA as described previously (14). We chose these high concentrations of POA to avoid selecting low-level resistance mutations in mas and in ppsA-E (which can be selected on 1 mM POA [14]). We observed spontaneous mutation frequencies of 10−4 (2 mM POA) and 10−5 (4 mM POA), consistent with the frequencies reported previously by us (14) and by Lanoix et al. (15). The frozen stocks were expanded in 7H9 broth and genomic DNA was extracted (16). To identify panD-independent POA resistance mechanisms, we picked a total of 21 POA-resistant strains from the 4 independent selection experiments and showed by targeted sequencing that 12 of the strains carried expected (11, 12, 14) panD resistance mutations, while the remaining 9 strains harbored wild-type panD genes. Targeted panD sequencing was carried out by PCR amplification of the panD locus as described in reference 11 using Phusion polymerase (Thermo Scientific) followed by capillary sequencing of the PCR product, performed by AIT Biotech, Singapore, using BigDye Terminator chemistry. The 12 panD mutation-containing POA-resistant strains were excluded from this study. We determined the MICs to POA of the 9 POA-resistant panD wild-type M. tuberculosis strains to confirm resistance in liquid culture, and they were found to display 4-fold increases in MIC50 values, indicating high levels of POA resistance similar to that of the representative panD mutant strain POAr 1 described previously in reference 14 (Table 1 and Fig. 1A). To verify that these strains displayed POA resistance specifically and not general antibiotic resistance, we measured MICs for rifampin and isoniazid and found that the strains showed wild-type-like susceptibility to these first-line TB drugs (Fig. 1B and C). We determined the MICs shown in Fig. 1A to C using the broth dilution method as described previously with minor modifications (4). The strains were grown to mid-log phase, spun down, and resuspended in fresh 7H9 medium adjusted to an optical density at 600 nm (OD600) of 0.1. Next, 100 μl of the cell suspension was added to wells containing 100 μl 2-fold serially diluted drugs in transparent flat-bottomed 96-well plates (Corning Costar) and sealed with Breathe-Easy membranes (Sigma-Aldrich). The plates were incubated for 7 days at 37°C with shaking at 80 rpm, and OD600 was measured using a spectrophotometer (Tecan Infinite M200 Pro). In addition to broth MICs, POA agar MICs were determined. The agar MIC was defined as the concentration of drug that suppressed colony formation when plating 104 CFU from mid-log cultures on 7H10 agar plates (in 3 independent experiments) and incubating for 3 weeks at 37°C as described previously (17). The 9 POA-resistant panD wild-type strains displayed at least 4-fold increases in agar MIC for POA compared with that of wild-type M. tuberculosis H37Rv (Table 1). Furthermore, we demonstrated that each of the 9 strains was also resistant to the prodrug PZA using the Bactec MGIT 960 PZA susceptibility test (18) and by determining PZA agar MICs (19) as shown in Table 1. Altogether, the broth and agar MICs of the POA-resistant panD wild-type strains for POA and PZA revealed that all 9 strains showed (i) resistance to the bioactive form of PZA (POA) and the prodrug PZA itself, (ii) similar resistance levels, and (iii) resistance levels similar to the resistance level of the previously identified panD mutant strain (14). In other words, the resistance levels were “high” compared with the low level of resistance caused by mutations in the polyketide synthases Mas and PpsA-E (14).
TABLE 1.
Sequence polymorphisms and POA and PZA broth and agar MICs of POA-resistant M. tuberculosis strains
| M. tuberculosis H37Rv straina | Mutations |
POAb |
PZAb |
|||
|---|---|---|---|---|---|---|
| clpC1c | Other genes | MIC50 (mM) in broth | MICd (mM) in agar | S/Re | MICd (mM) in agar | |
| Wild-type | —f | — | 1.5 | 1 | S | 2 |
| POAr 1 (panD1)g I | — | panD: Δ380A | 6.0 | >4 | R | >4 |
| POAr 11 (clpC1-1) 1, I | G-10Ch | — | 5.5 | >4 | R | >4 |
| POAr 12 (clpC1-2) 2, II | C262G/Leu88Val | mmpL7: T534G/Asp178Glu | 5.5 | >4 | R | >4 |
| POAr 13 (clpC1-3) 3, I | G296A/Gly99Asp | — | 6.0 | >4 | R | >4 |
| POAr 14 (clpC1-3) 4, I | G296A/Gly99Asp | — | 6.0 | >4 | R | >4 |
| POAr 15 (clpC1-4) 4, II | T323C/Ile108Thr | — | 6.5 | >4 | R | >4 |
| POAr 16 (clpC1-5) 3, I | G341T/Arg114Leu | — | 5.0 | >4 | R | >4 |
| POAr 17 (clpC1-6) 1, II | C577G/Arg193Gly | Rv3626c: G710T/Arg237Leu | 6.0 | >4 | R | >4 |
| POAr 18 (clpC1-7) 2, II | A625G/Lys209Glu | — | 6.0 | >4 | R | >4 |
| POAr 19 (clpC1-8) 3, I | T866C/Leu289Pro | ppe47: Ins14G; yrbE4B: G715A/Gly239Arg | 6.0 | >4 | R | >4 |
Mutants were isolated from four independent batches of bacterial cultures: 1 and 2, selected on Middlebrook 7H10 agar containing 0.5% glycerol; 3 and 4, selected on Middlebrook 7H10 agar without glycerol; I, mutants were selected on agar containing 2 mM POA; II, mutants were selected with 4 mM POA.
Drug susceptibility tests were carried out 3 times independently and mean values are shown.
Polymorphisms were identified by whole-genome sequencing and verified by targeted sequencing as described in the text.
Maximum concentration of drug tested was 4 mM.
BACTEC MGIT 960 test for susceptibility (S) or resistance (R) to 100 μg/ml PZA.
—, not applicable.
Isolated and described in reference 14.
The polymorphism is 10 bp upstream of the clpC1 start codon in the transcribed but untranslated leader sequence (see Fig. 1E).
FIG 1.
Characterization of pyrazinoic acid (POA)-resistant panD wild-type M. tuberculosis strains. Growth inhibition dose-response curves of 9 POA-resistant panD wild-type strains, POAr 11 to 19, POA-sensitive wild-type M. tuberculosis H37Rv, and a representative POA-resistant panD mutant strain, POAr 1, isolated previously (14), for (A) POA, (B) rifampin (RIF), and (C) isoniazid (INH). Experiments were carried out 3 times independently with technical replicates. Mean values and standard deviations from results of representative experiments are shown. (D) Location of 7 ClpC1 amino acid sequence polymorphisms in POA-resistant panD wild-type M. tuberculosis strains POAr 12 to 19. ClpC1 domain organization is shown as described in reference 23. Within the N-terminal domain, two repeats are labeled I and II. A and B in the D1 and D2 domains indicate Walker A and Walker B motifs, respectively. (E) Location of the nucleotide sequence polymorphism G to C (−10) in the untranslated leader mRNA of clpC1 in POA-resistant panD wild-type M. tuberculosis strain POAr 11. The organization of the clpC1 upstream region is shown as described in reference 20. A conserved TANNNT promoter motif (TACAGT) and the transcriptional start site (TSS), located 55 bp upstream of the clpC1 coding sequence, are indicated (20). Refer to Table 1 for genotypes and phenotypes of strains.
To identify the genomic polymorphisms associated with resistance, the 9 POA/PZA-resistant panD wild-type strains were subjected to whole-genome sequencing. Whole-genome sequencing was performed on Illumina MiSeq as described previously (14). As was expected from selecting for resistance on agar containing high POA concentrations, we did not detect low POA resistance conferring mas or ppsA-E mutations (14) in the 9 strains. Table 1 shows that 8 of the 9 strains (POAr 12 to 19) carried nonsynonymous single nucleotide polymorphisms in the coding sequences of ClpC1 (Rv3596c). These 8 clpC1 missense mutation-harboring strains presented 7 different amino acid substitutions in the N-terminal and D1 domains of this 848-amino-acid protein (Fig. 1D), with one pair carrying identical amino acid changes (Table 1, POAr 13 and 14). As the members of this pair were isolated from different selection experiments (i.e., from independently grown cultures), they likely represent independent mutational events and are not clonal in nature. The remaining POA-resistant strain, POAr 11, showed a nucleotide polymorphism 10 bp upstream of the ClpC1 encoding sequence in the leader mRNA of the transcript (Table 1 and Fig. 1E) (20). Whether this mutation affects the expression level of the ClpC1 protein remains to be determined. The mutations in the clpC1 gene were confirmed by targeted PCR sequencing using the following primer pairs: 5′-CGGCGACCTGACATTTGGCTACC-3′ and 5′-ACGCCTTCCCCTTCATGGATCAGG-3′ for strain POAr 11 carrying a mutation upstream of ClpC1 encoding sequence, and 5′-ACATATGTTCGAACGATTTACCGACCGTGC-3′ and 5′-TGAATTCACCCATGTCAATCTGAATAAGCGC-3′ for the remaining strains with mutations in the ClpC1-encoding region. Taken together, all 9 POA/PZA-resistant panD wild-type M. tuberculosis strains harbored nucleotide polymorphisms in the clpC1 locus. This result suggests that the observed mutations in this gene cause POA/PZA resistance.
Caseinolytic protein C (ClpC) can be found in both prokaryotes and eukaryotes. ClpC belongs to class I of the AAA+ (ATPases associated with a variety of cellular activities) superfamily containing one N-terminal and two nucleotide-binding domains (D1 and D2), the latter harboring the Walker A and Walker B motifs required for ATP binding and hydrolysis (21) (Fig. 1D). Bacterial ClpC proteins have been reported to function as molecular chaperones and specificity factors involved in determining substrates to be degraded by the caseinolytic protease complex (22). Similarly, in M. tuberculosis, the ClpC homolog ClpC1 self-associates to form oligomers displaying ATPase and molecular chaperone activities in vitro (23). ClpC1 works as an unfoldase in concert with the proteases ClpP1 and ClpP2 of the caseinolytic protease complex (24), and it was demonstrated that this degradative protease is essential for the viability of M. tuberculosis (25). Due to the critical role of this protease in survival and virulence, different components of this complex have been proposed as attractive therapeutic targets (26). Our POA-resistant strains harbor missense mutations in 2 different regions of the ClpC1 protein. We observed 4 different missense mutations in the N-terminal domain of ClpC1, with 3 located in the N-terminal repeat II (as annotated by reference 23) and the fourth mutation just outside this repeat (Fig. 1D). While the exact role of the N-terminal domain of M. tuberculosis ClpC1 is unclear, it is interesting to note that it acts as the binding site of several novel antimycobacterials, including cyclomarin (27), lassomycin (28), and ecumicin (29). In other prokaryotes, the N-terminal domain of ClpC is the site for interacting with adaptor proteins, either acting as the binding site or aiding in substrate recognition (21, 30). The other 3 missense mutations are located in the D1 domain, flanking the Walker A and Walker B motifs (Fig. 1D).
It remains to be determined whether the observed missense mutations in the coding regions of clpC1 cause POA/PZA resistance via a direct mechanism, for instance, by preventing binding of the drug to the ClpC1 protein, or an indirect mechanism, for instance, by affecting the substrate selectivity of the ClpC1 unfoldase and therefore the level of some proteins targeted for degradation by the caseinolytic protease complex.
Similar to the POA/PZA-associated resistance mutations in panD isolated in vitro (11, 12, 14), clpC1 polymorphisms appear to not be strongly associated with PZA resistance in clinical isolates of M. tuberculosis. In the Genome-wide Mycobacterium tuberculosis variation (GMTV) database (31), we did not find any strains with the clpC1 polymorphisms observed in our POA-resistant strains. It has been shown that clpC1 is essential for growth in vitro (32, 33) and for survival within macrophages (34). Whether the absence of our POA/PZA resistance-causing mutations in clinical isolates results from a loss of in vivo fitness is under investigation in mouse infection studies.
In conclusion, we add here to the growing list of POA/PZA candidate targets and resistance mechanisms, including fatty acid synthetase I (FASI), ribosomal protein S1 (RpsA), the aspartate decarboxylase PanD, and the polyketide synthases Mas and PpsA-E, by demonstrating that missense mutations in the unfoldase/ATPase ClpC1 of the caseinolytic protease complex are associated with POA and PZA resistance. This provides further support for a working model suggesting that the excellent sterilizing activity of PZA may be due, in part, to it being a “dirty drug”, i.e., this small “fragment-like” antimycobacterial can hit multiple targets and pathways inside the tubercle bacillus (35).
ACKNOWLEDGMENTS
This research was supported by the Singapore Ministry of Health's National Medical Research Council under its TCR Flagship (no. NMRC/TCR/011-NUHS/2014) and Centre MINE (research core number 4, NMRC/CG/013/2013) grants to T.D. and is part of Singapore Programme of Research Investigating New Approaches to Treatment of Tuberculosis (SPRINT-TB), managed by K. Rutkute and led by N. Paton. We thank S. Phyu and M. Gengenbacher and the Yong Loo Lin School of Medicine BSL3 core facility for support. P.G. received a scholarship from the Yong Loo Lin School of Medicine.
M. Yee and P. Gopal performed the experiments and analyzed the data. M. Yee, P. Gopal, and T. Dick wrote the manuscript.
The authors declare no competing interests that might be perceived to influence the results and discussion stated in this paper.
REFERENCES
- 1.Mitchison DA. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 2.Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, McHugh TD, Schito M, Maeurer M, Nunn AJ. 2014. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 14:327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Shi W, Zhang W, Mitchison D. 2013. Mechanisms of pyrazinamide action and resistance. Microbiol Spectr 2:1–12. doi: 10.1128/microbiolspec.MGM2-0023-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Via LE, Savic R, Weiner DM, Zimmerman MD, Prideaux B, Irwin SM, Lyon E, O'Brien P, Gopal P, Eum S, Lee M, Lanoix J-P, Dutta NK, Shim T, Cho JS, Kim W, Karakousis PC, Lenaerts A, Nuermberger E, Barry CE, Dartois V. 2015. Host-mediated bioactivation of pyrazinamide: implications for efficacy, resistance, and therapeutic alternatives. ACS Infect Dis 1:203–214. doi: 10.1021/id500028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scorpio A, Zhang Y. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med 2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Scorpio A, Nikaido H, Sun Z. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol 181:2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang H, Sun Z. 2003. Susceptibility of Mycobacterium tuberculosis to weak acids. J Antimicrob Chemother 52:56–60. doi: 10.1093/jac/dkg287. [DOI] [PubMed] [Google Scholar]
- 8.Peterson ND, Rosen BC, Dillon NA, Baughn AD. 2015. Uncoupling environmental pH and intrabacterial acidification from pyrazinamide susceptibility in Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:7320–7326. doi: 10.1128/AAC.00967-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimhony O, Vilchèze C, Arai M, Welch JT, Jacobs WR. 2007. Pyrazinoic acid and its n-propyl ester inhibit fatty acid synthase type I in replicating tubercle bacilli. Antimicrob Agents Chemother 51:752–754. doi: 10.1128/AAC.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE, Wang H, Zhang W, Zhang Y. 2011. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi W, Chen J, Feng J, Cui P, Zhang S, Weng X, Zhang W, Zhang Y. 2014. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in Mycobacterium tuberculosis. Emerg Microbes Infect 3:e58. doi: 10.1038/emi.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Chen J, Shi W, Liu W, Zhang W, Zhang Y. 2013. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect 2:e34. doi: 10.1038/emi.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon NA, Peterson ND, Rosen BC, Baughn AD. 2014. Pantothenate and pantetheine antagonize the antitubercular activity of pyrazinamide. Antimicrob Agents Chemother 58:7258–7263. doi: 10.1128/AAC.04028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopal P, Yee M, Sarathy J, Low JL, Sarathy JP, Kaya F, Dartois V, Gengenbacher M, Dick T. 2016. Pyrazinamide resistance is caused by two distinct mechanisms: prevention of coenzyme A depletion and loss of virulence factor synthesis. ACS Infect Dis 2:616–626. doi: 10.1021/acsinfecdis.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanoix J-P, Tasneen R, O'Brien P, Sarathy J, Safi H, Pinn M, Alland D, Dartois V, Nuermberger E. 2016. High systemic exposure of pyrazinoic acid has limited antituberculosis activity in murine and rabbit models of tuberculosis. Antimicrob Agents Chemother 60:4197–4205. doi: 10.1128/AAC.03085-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Käser M, Ruf M-T, Hauser J, Marsollier L, Pluschke G. 2009. Optimized method for preparation of DNA from pathogenic and environmental mycobacteria. Appl Environ Microbiol 75:414–418. doi: 10.1128/AEM.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopal P, Dick T. 2015. The new tuberculosis drug Perchlozone shows cross-resistance with thiacetazone. Int J Antimicrob Agents 45:430–433. doi: 10.1016/j.ijantimicag.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqi SH, Rüsch-Gerdes S. 2006. MGIT procedure manual. Foundation for Innovative New Diagnostics, Geneva, Switzerland. [Google Scholar]
- 19.Heifets L, Sanchez T. 2000. New agar medium for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol 38:1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes T, Schubert OT, Rose G, Arnvig KB, Comas I, Aebersold R, Young DB. 2013. Genome-wide mapping of transcriptional start sites defines an extensive leaderless transcriptome in Mycobacterium tuberculosis. Cell Rep 5:1121–1131. doi: 10.1016/j.celrep.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dougan DA, Mogk A, Zeth K, Turgay K, Bukau B. 2002. AAA+ proteins and substrate recognition, it all depends on their partner in crime. FEBS Lett 529:6–10. doi: 10.1016/S0014-5793(02)03179-4. [DOI] [PubMed] [Google Scholar]
- 22.Wawrzynow A, Banecki B, Zylicz M. 1996. The Clp ATPases define a novel class of molecular chaperones. Mol Microbiol 21:895–899. doi: 10.1046/j.1365-2958.1996.421404.x. [DOI] [PubMed] [Google Scholar]
- 23.Kar NP, Sikriwal D, Rath P, Choudhary RK, Batra JK. 2008. Mycobacterium tuberculosis ClpC1. FEBS J 275:6149–6158. doi: 10.1111/j.1742-4658.2008.06738.x. [DOI] [PubMed] [Google Scholar]
- 24.Akopian T, Kandror O, Raju RM, Unnikrishnan M, Rubin EJ, Goldberg AL. 2012. The active ClpP protease from M. tuberculosis is a complex composed of a heptameric ClpP1 and a ClpP2 ring. EMBO J 31:1529–1541. doi: 10.1038/emboj.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju RM, Jedrychowski MP, Wei J-R, Pinkham JT, Park AS, O'Brien K, Rehren G, Schnappinger D, Gygi SP, Rubin EJ. 2014. Post-translational regulation via Clp protease is critical for survival of Mycobacterium tuberculosis. PLoS Pathog 10:e1003994. doi: 10.1371/journal.ppat.1003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raju RM, Goldberg AL, Rubin EJ. 2012. Bacterial proteolytic complexes as therapeutic targets. Nat Rev Drug Discov 11:777–789. doi: 10.1038/nrd3846. [DOI] [PubMed] [Google Scholar]
- 27.Vasudevan D, Rao SPS, Noble CG. 2013. Structural basis of mycobacterial inhibition by cyclomarin A. J Biol Chem 288:30883–30891. doi: 10.1074/jbc.M113.493767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavrish E, Sit CS, Cao S, Kandror O, Spoering A, Peoples A, Ling L, Fetterman A, Hughes D, Bissell A, Torrey H, Akopian T, Mueller A, Epstein S, Goldberg A, Clardy J, Lewis K. 2014. Lassomycin, a ribosomally synthesized cyclic peptide, kills Mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2. Chem Biol 21:509–518. doi: 10.1016/j.chembiol.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao W, Kim J-Y, Anderson JR, Akopian T, Hong S, Jin Y-Y, Kandror O, Kim J-W, Lee I-A, Lee S-Y, McAlpine JB, Mulugeta S, Sunoqrot S, Wang Y, Yang S-H, Yoon T-M, Goldberg AL, Pauli GF, Suh J-W, Franzblau SG, Cho S. 2015. The cyclic peptide ecumicin targeting ClpC1 is active against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother 59:880–889. doi: 10.1128/AAC.04054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirstein J, Schlothauer T, Dougan DA, Lilie H, Tischendorf G, Mogk A, Bukau B, Turgay K. 2006. Adaptor protein controlled oligomerization activates the AAA+ protein ClpC. EMBO J 25:1481–1491. doi: 10.1038/sj.emboj.7601042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chernyaeva EN, Shulgina MV, Rotkevich MS, Dobrynin PV, Simonov SA, Shitikov EA, Ischenko DS, Karpova IY, Kostryukova ES, Ilina EN, Govorun VM, Zhuravlev VY, Manicheva OA, Yablonsky PK, Isaeva YD, Nosova EY, Mokrousov IV, Vyazovaya AA, Narvskaya OV, Lapidus AL, O'Brien SJ. 2014. Genome-wide Mycobacterium tuberculosis variation (GMTV) database: a new tool for integrating sequence variations and epidemiology. BMC Genomics 15:308. doi: 10.1186/1471-2164-15-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 33.Griffin JE, Gawronski JD, DeJesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rengarajan J, Bloom BR, Rubin EJ. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102:8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopal P, Dick T. 2014. Reactive dirty fragments: implications for tuberculosis drug discovery. Curr Opin Microbiol 21:7–12. doi: 10.1016/j.mib.2014.06.015. [DOI] [PubMed] [Google Scholar]