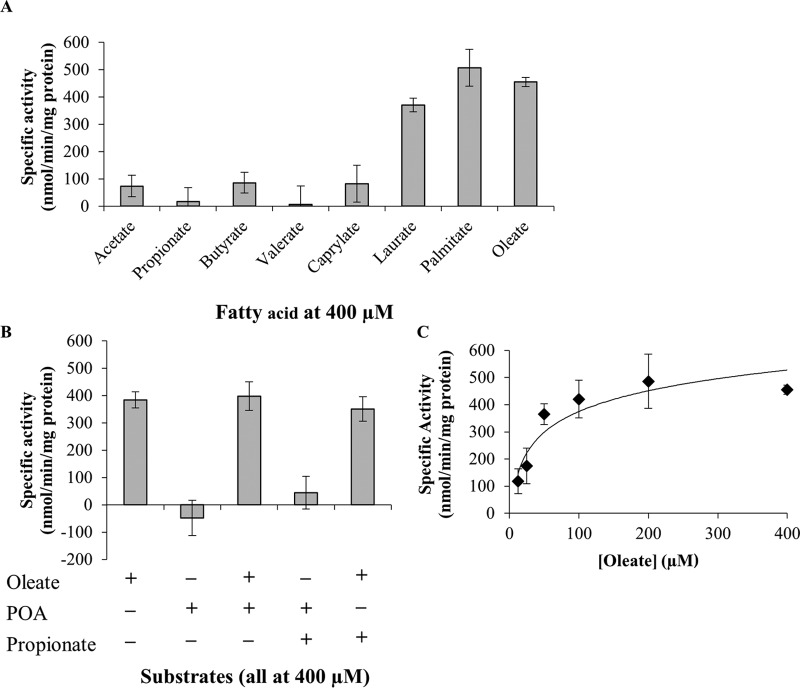
FIG 4.
Enzymatic characterization of the M. tuberculosis FadD2. (A) Fatty acid substrate specificity of FadD2; (B) additional assays conducted to assess the allosteric modulation of FadD2 and use of POA as the substrate; (C) kinetic profile of FadD2 with oleate as the substrate. Recombinant histidine-tagged FadD2 was propagated in E. coli and purified by nickel affinity chromatography. Enzymatic assays were conducted using 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB; or Ellman's reagent) to monitor CoA-SH consumption at 412 nm. Specific activities were computed using the previously determined extinction coefficient for DTNB of 13,600 M−1 cm−1. All specific activity values shown are equivalent to the average reaction velocity over the initial 5-min incubation period. Error bars depict the standard deviations from three assays.