ABSTRACT
Novel therapies for methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection (BSI) are needed in the setting of reduced antibiotic susceptibilities and therapeutic failure. Ceftaroline is a cephalosporin antibiotic with MRSA activity. Although not FDA approved for MRSA BSI, ceftaroline has generated much interest as a potential treatment option. However, detailed descriptions of its use in this setting remain limited. To address this, we conducted a retrospective, multicenter, observational study of adult patients with MRSA BSI treated with at least 72 h of ceftaroline from 2011 to 2015. Safety outcomes were examined in the overall cohort, while efficacy outcomes were examined among patients who had not cleared their BSI prior to ceftaroline initiation. Data were also stratified by ceftaroline monotherapy or combination therapy. Predictors of clinical failure on ceftaroline treatment were also sought. Overall, 211 patients were included in the safety population; Clostridium difficile infection, rash, and neutropenia occurred in 6 patients (2.8%), 7 patients (3.3%), and 3 patients (1.4%), respectively. Clinical success was observed in 86 (68.3%) of the 126 patients included in the efficacy population. The monotherapy and combination therapy subgroups had similar proportions of patients experiencing success (69.7 and 64.9%, respectively). The median BSI durations post-ceftaroline treatment were 2 days (interquartile range, 1 to 4 days) for monotherapy and 3 days (interquartile range, 1.5 to 5 days) for combination therapy. Higher acute physiology and chronic health evaluation II scores and comorbid malignancy independently predicted treatment failure. Ceftaroline appears effective for MRSA BSI as both monotherapy and combination therapy. However, comparative studies are needed to further delineate the role of ceftaroline in MRSA BSI treatment.
KEYWORDS: MRSA, bacteremia, treatment failure, infective endocarditis, pneumonia, bone and joint infection, beta-lactam, vancomycin, daptomycin
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) continues to be a tremendous public health issue, causing a range of infections in both community and health care settings (1). MRSA is estimated to result in more than 80,000 invasive infections and 11,000 deaths in the United States annually (1). Bloodstream infections (BSIs) caused by MRSA have been associated with substantial morbidity, mortality, and health care costs (2–4). Although vancomycin is considered the treatment of choice for MRSA BSI, treatment failure, the emergence of reduced-vancomycin-susceptibility phenotypes such as heterogeneous vancomycin-intermediate Staphylococcus aureus, and adverse drug reactions necessitate alternative treatment options. (5–7) Daptomycin is currently the primary alternative to vancomycin for MRSA BSI, but its use is not without limitations (5, 8–11). Interactions with pulmonary surfactant render daptomycin ineffective for BSI secondary to a respiratory source (12). Daptomycin-nonsusceptible Staphylococcus aureus has also emerged, along with descriptions of daptomycin failure following vancomycin failure (13–15). Collectively, the limitations of these two standard-of-care agents for MRSA BSI demonstrate the urgent need for additional evidence-based antibiotic therapies.
Ceftaroline fosamil, the prodrug of ceftaroline, is a cephalosporin antibiotic with potent bactericidal Gram-positive activity (16). To date, it is the only beta-lactam antibiotic commercially available in the United States with inherent activity against MRSA. Although not FDA labeled for Staphylococcus aureus BSI, ceftaroline fosamil, here called ceftaroline, has garnered considerable interest for the treatment of MRSA BSI (17). However, currently reported data examining the effectiveness and safety of ceftaroline specific for MRSA BSI are limited. Most investigations are limited by small sample sizes and frequently describe the use of ceftaroline in combination with other MRSA-active therapies for treatment-refractory BSI (17–24). Therefore, this analysis sought to provide a detailed, multicenter description of the clinical characteristics and outcomes of patients with MRSA BSI treated with ceftaroline as monotherapy or combination therapy. As a secondary objective, we also sought to explore factors independently associated with clinical failure among patients treated with ceftaroline for MRSA BSI.
RESULTS
In total, 211 patients received ceftaroline for ≥72 h for MRSA BSI during the study period and were included in the safety population. A complete description of patient baseline demographics and clinical characteristics of this population are displayed in Table S1 in the supplemental material. Ceftaroline was administered every 8 h in 108 (51.2%) patients and was given in combination with another MRSA-active antibiotic in 46 (21.8%) patients. The median inpatient duration of ceftaroline therapy was 11 days (interquartile range [IQR], 5 to 15 days). Inpatient adverse reactions were uncommon, with Clostridium difficile infection, rash, and neutropenia occurring in 6 patients (2.8%), 7 patients (3.3%), and 3 patients (1.4%), respectively. Among the three patients experiencing neutropenia, two were receiving 600 mg ceftaroline every 8 h, while one was receiving 400 mg every 12 h. The times from ceftaroline initiation to neutropenia onset were 13, 20, and 15 days, respectively.
Patient characteristics and outcomes of the 126 patients included in the efficacy population are shown in Table 1. The most common MRSA BSI sources were lower respiratory tract (32.5%) and infective endocarditis (24.6%). Ceftaroline was the second (54.0%) or third (35.7%) directed therapy in a majority of cases, and the median BSI duration pre-ceftaroline treatment was 3 days (IQR, 2.0 to 6.3 days). The most common reasons for ceftaroline use were perceived failure of prior therapy as documented by the treating physician (53.2%) and elevated vancomycin MIC (19.8%). Clinical success was observed for 86 (68.3%) of the patients in the efficacy population, with 28 (22.2%) experiencing in-hospital mortality.
TABLE 1.
Baseline demographics, clinical characteristics, and outcomes of the efficacy population
| Parameter | Value for group |
||
|---|---|---|---|
| Overall (n = 126) | Monotherapy (n = 89) | Combination therapy (n = 37) | |
| Demographics | |||
| Median age (yr) (IQR) | 59 (45.5–66.8) | 59 (48–67) | 57 (45–66.5) |
| No. of male patients (%) | 70 (55.6) | 50 (56.2) | 20 (54.1) |
| No. of patients of race (%) | |||
| African American | 86 (68.3) | 62 (69.7) | 24 (64.9) |
| Caucasian | 34 (27.0) | 21 (23.6) | 13 (35.1) |
| Hispanic/Latino | 1 (0.8) | 1 (1.1) | 0 |
| Other | 5 (4.0) | 5 (5.6) | 0 |
| No. of patients in hospital system (%) | |||
| Detroit Medical Center | 83 (65.9) | 52 (58.4) | 31 (83.8) |
| UF Health-Shands Hospital | 30 (23.8) | 26 (29.2) | 4 (10.8) |
| Henry Ford Hospital | 13 (10.3) | 11 (12.4) | 2 (5.4) |
| Comorbidities and past medical history | |||
| No. of patients with comorbidity or past medical history (%) | |||
| Prior antibiotics (90 days) | 33 (26.2) | 24 (27.0) | 9 (24.3) |
| Prior MRSA infection (1 yr) | 20 (15.9) | 14 (15.7) | 6 (16.2) |
| Obesity | 48 (38.1) | 37 (41.6) | 11 (29.7) |
| Diabetes mellitus | 47 (37.3) | 34 (38.2) | 13 (35.1) |
| Chronic kidney disease | 34 (27.0) | 25 (28.1) | 9 (24.3) |
| Chronic hemodialysis | 26 (20.6) | 19 (21.3) | 7 (18.9) |
| Liver disease | 20 (15.9) | 13 (14.6) | 7 (18.9) |
| Intravenous drug user | 24 (19.0) | 14 (15.7) | 10 (27.0) |
| Malignancy | 7 (5.6) | 4 (4.5) | 3 (8.1) |
| HIV/AIDS | 8 (6.3) | 3 (3.4) | 5 (13.5) |
| Neutropeniaa | 3 (2.4) | 2 (2.2) | 1 (2.7) |
| Median Charlson comorbidity index (IQR) | 3 (2–5) | 3 (2–5) | 3 (2–5) |
| Clinical characteristics | |||
| No. of patients in intensive care unit (%)b | 45 (35.7) | 31 (34.8) | 14 (37.8) |
| Median APACHE II score (IQR)b | 16 (12–22) | 16 (12–22) | 16 (13–22) |
| No. (%) of patients with: | |||
| Lower respiratory tract source | 41 (32.5) | 30 (33.7) | 11 (29.7) |
| Infective endocarditis source | 31 (24.6) | 20 (22.5) | 11 (29.7) |
| Bone/joint source | 26 (20.6) | 20 (22.5) | 6 (16.2) |
| Intravenous catheter source | 20 (15.9) | 16 (18.0) | 4 (10.8) |
| Skin/soft tissue source | 11 (8.7) | 9 (10.1) | 2 (5.4) |
| Other source | 22 (17.5) | 14 (15.7) | 8 (21.6) |
| Polymicrobial BSI | 10 (7.9) | 6 (6.7) | 4 (10.8) |
| Vancomycin-susceptible strainc | 125 (99.2) | 88 (98.9) | 37 (100.0) |
| Daptomycin-susceptible strainc,d | 116 (96.7) | 80 (96.4) | 36 (97.3) |
| Ceftaroline-susceptible straine | 94 (96.9) | 70 (95.9) | 24 (100.0) |
| Treatment information | |||
| No. (%) of patients with: | |||
| Infectious diseases consultf | 113 (93.4) | 79 (91.9) | 34 (97.1) |
| Source control pursuedg | 42 (34.7) | 28 (32.9) | 14 (38.9) |
| Prior directed therapy with vancomycin | 107 (84.9) | 74 (83.1) | 33 (89.2) |
| Prior directed therapy with daptomycin | 48 (38.1) | 22 (24.7) | 26 (70.3) |
| Ceftaroline dosing frequency | |||
| Every 8 h | 66 (52.4) | 52 (58.4) | 14 (37.8) |
| Every 12 h | 54 (42.9) | 33 (37.1) | 21 (56.8) |
| Every 24 h | 6 (4.8) | 4 (4.5) | 2 (5.4) |
| Ceftaroline dose | |||
| 600 mg | 76 (60.3) | 55 (61.8) | 21 (56.8) |
| 400 mg | 19 (15.1) | 14 (15.7) | 5 (13.5) |
| 300 mg | 11 (8.7) | 8 (9.0) | 3 (8.1) |
| 200 mg | 20 (15.9) | 12 (13.5) | 8 (21.6) |
| Median ceftaroline inpatient duration (days) (IQR) | 13 (5–21) | 13 (5–22) | 14 (4–17) |
| No. (%) of patients receiving combination therapy with: | |||
| Daptomycin | 28 (22.2) | 28 (75.7) | |
| Vancomycin | 3 (2.4) | 3 (8.1) | |
| Gentamicin | 3 (2.4) | 3 (8.1) | |
| Rifampin | 5 (4.0) | 5 (13.5) | |
| Outcomes | |||
| No. of patients achieving clinical success (%) | 86 (68.3) | 62 (69.7) | 24 (64.9) |
| No. of patients with in-hospital mortality (%) | 28 (22.2) | 17 (19.1) | 11 (29.7) |
| No. of patients with cleared BSI on ceftaroline (%) | 115 (91.3) | 79 (88.8) | 36 (97.3) |
| Median BSI duration post-ceftaroline initiation (days) (IQR) | 3 (1–4) | 2 (1–4) | 3 (1.5–5) |
| Median length of stay post-ceftaroline initiation (days) (IQR) | 12 (8–20) | 12 (8–18) | 15 (8.5–22.5) |
Defined as an absolute neutrophil count of <500 cells/mm3 at ceftaroline initiation.
At the time of the index culture.
Susceptibility determined by Microscan, Vitek 2, BD Phoenix, or Etest.
Daptomycin susceptibility were available for 120, 83, and 37 patients in the efficacy population and the monotherapy and combination therapy subgroups, respectively.
Ceftaroline susceptibility was determined by Etest for 97, 73, and 24 patients in the efficacy population and the monotherapy and combination therapy subgroups, respectively.
Infectious diseases consult status known for 121, 86, and 35 patients in the efficacy population and the monotherapy and combination therapy subgroups, respectively.
Pursuit of source control known for 121, 85, and 36 patients in the efficacy population and the monotherapy and combination therapy subgroups, respectively.
Table 1 also displays the characteristics and outcomes of the monotherapy and combination therapy subgroups within the efficacy population. Patient characteristics in these subgroups were largely similar to those of the overall cohort. The most common BSI source for the 89 patients receiving monotherapy was lower respiratory tract (35.2%), whereas infective endocarditis and lower respiratory tract (29.7% each) were most common among the 37 combination therapy patients. Ceftaroline monotherapy was predominantly used as the second directed therapy (54.0%), with a median BSI duration pre-ceftaroline treatment of 3 days (IQR, 2 to 5.5 days). In contrast, ceftaroline combination therapy was the third directed regimen in most cases (69.6%), with a corresponding median BSI duration pre-ceftaroline treatment of 4 days (IQR, 2.5 to 9 days). Daptomycin was the most common combination agent, received by 33 (71.7%) of the patients in the combination therapy subgroup. Clinical success was observed in 62 patients (69.7%) and 24 patients (64.9%) in the monotherapy and combination therapy groups, respectively. Bloodstream infection clearance with ceftaroline monotherapy occurred in 79 (88.8%) patients, with a median BSI duration post-ceftaroline treatment of 2 days (IQR, 1 to 4 days). Among patients in the combination therapy group, clearance with ceftaroline occurred in 36 (97.3%) patients, and the median BSI duration post-ceftaroline treatment was 3 days (IQR, 1.5 to 5 days). The time to BSI clearance post-ceftaroline initiation in both the monotherapy and combination therapy subgroups is plotted in Fig. 1.
FIG 1.
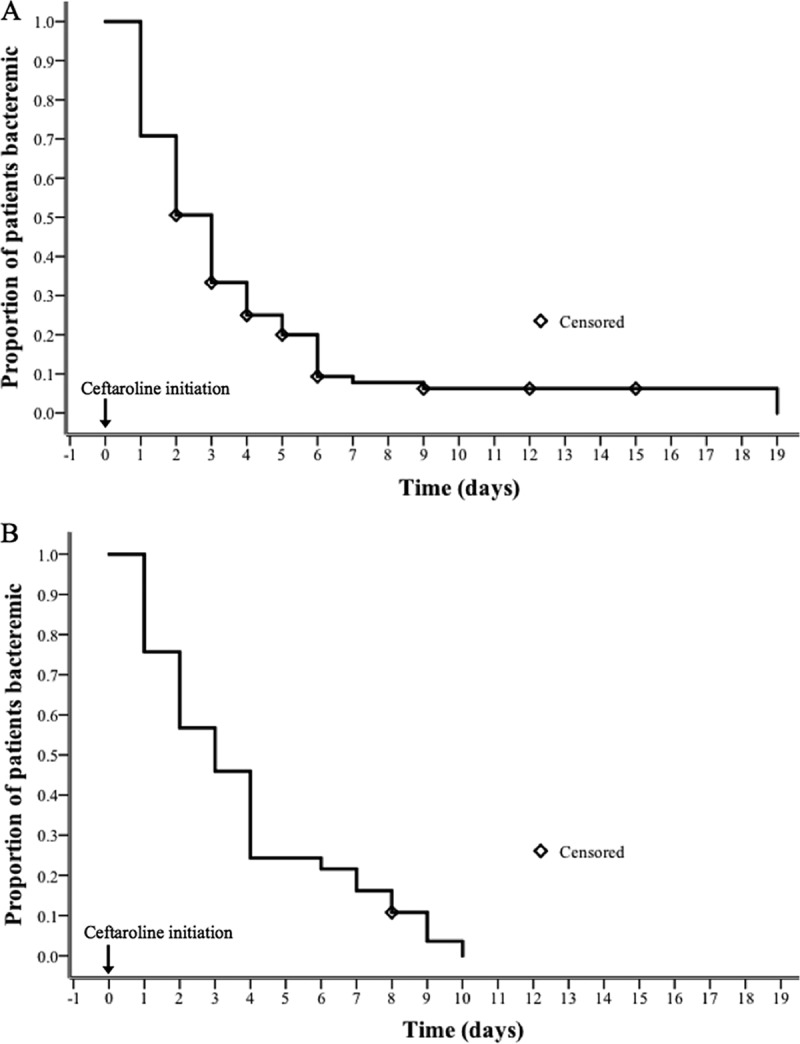
Kaplan-Meier curves for the time to clearance of bloodstream infection post-ceftaroline initiation in the efficacy population. (A) Ceftaroline monotherapy; (B) ceftaroline combination therapy.
Bivariate comparisons between patients experiencing success and those experiencing failure in the efficacy population are shown in Table S2 in the supplemental material. No difference in ceftaroline MICs was noted between those experiencing success or failure when ceftaroline MIC was modeled continuously (median, 0.5 mg/liter [IQR, 0.5 to 1 mg/liter] versus 0.5 mg/liter [IQR, 0.5 to 1 mg/liter]; P = 0.621) or ordinally (see Fig. S1 in the supplemental material). Also, the proportion of patients experiencing clinical success did not vary across ceftaroline dosing frequencies overall or when stratified by renal function (Table S3). Based on bivariate analyses, acute physiology and chronic health evaluation II (APACHE II) score, comorbid malignancy, lower respiratory tract BSI source, bone/joint BSI source, and ceftaroline dose in milligrams were entered into the multivariable logistic regression model as factors associated with failure. The results of the final multivariable logistic regression model are listed in Table 2. APACHE II score and malignancy were independently associated with failure.
TABLE 2.
Multivariable logistic regression analysis of factors independently associated with treatment failure with ceftaroline in the efficacy populationa
| Variable | Odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| APACHE II score | 1.100 (1.037–1.166) | 1.093 (1.044–1.145) |
| Malignancy | 6.000 (1.111–32.405) | 3.127 (1.009–9.686) |
| Lower respiratory tract source | 2.632 (1.198–5.783) | |
| Bone/joint source | 0.442 (0.153–1.274) | |
| Ceftaroline dose | ||
| 600 mg | ||
| 400 mg | 3.856 (1.350–11.017) | |
| 300 mg | 2.892 (0.785–10.651) | |
| 200 mg | 2.314 (0.814–6.577) |
P = 0.574 as determined by a Hosmer-Lemeshow goodness-of-fit test and a variance inflation factor of <3 for all variables included for model entry. CI, confidence interval.
DISCUSSION
Given the widespread burden of MRSA BSI on the health care system and the limitations of current standard therapies, this analysis sought to assess ceftaroline as a potential treatment option. The results suggest the ceftaroline is effective and safe for MRSA BSI when given as monotherapy or in combination with other MRSA-active antibiotics. Clinical success was observed in nearly 70% of patients, and BSI clearance while receiving ceftaroline occurred in more than 90% of patients. Ceftaroline was the second or third directed therapy in the vast majority of included patients, and perceived failure of prior therapy was the documented reason for ceftaroline use in more than half of these patients. Thus, these results primarily represent ceftaroline as “salvage” therapy.
These results are consistent with data from previous observational studies of MRSA BSI treated with ceftaroline, the majority of which demonstrated success in 70% of cases or more, with a limited number of adverse effects (17–21, 23, 24). Due to the robust sample size, the present study is able to build upon previously reported observational data by evaluating the outcomes of patients receiving ceftaroline monotherapy and combination therapy separately. Although direct comparison with standard MRSA BSI therapies is necessary before a firm conclusion on the efficacy of ceftaroline can be drawn, crude comparisons with data from previous studies place these results into context. Despite the fact that ceftaroline was the second or third directed therapy in most patients in this study, the observed clinical success and mortality rates compare favorably to those for vancomycin (6). This echoes the findings of a small comparative analysis of ceftaroline and vancomycin treatment for MRSA BSI (23). Comparison with data from previous daptomycin studies is challenging due to the exclusion of BSI secondary to pneumonia from these studies (9–11). One-third of this cohort was comprised of patients with a lower respiratory tract source of infection, which carries a high risk of mortality (25, 26). Nonetheless, the proportion of patients who experienced failure was similar to that in a recently reported evaluation of daptomycin for MRSA BSI (11). Collectively, these findings suggest that ceftaroline has promise for MRSA BSI treatment.
Another notable finding is the time to BSI clearance observed following ceftaroline initiation. Clearance following the initiation of ceftaroline as monotherapy or in combination occurred in 50% of patients within 3 days and in more than 80% of patients within 7 days. This finding is consistent with data from previous ceftaroline observational studies and resembles the time to MSSA BSI clearance by beta-lactams (22–24). Clinical data suggest that beta-lactams provide quicker BSI clearance than does vancomycin for staphylococcal BSI (8, 27). A potential explanation for this finding is the ability of beta-lactams, including ceftaroline, to sensitize Staphylococcus aureus to killing by human cationic host defense peptides (HDPs) of the innate immune system (24, 28, 29). This interaction is likely mediated by enhanced cationic HDP cell membrane binding through increased membrane negativity and is analogous to the mechanism of synergy observed between daptomycin and ceftaroline (29, 30).
This analysis also explored factors associated with treatment failure. Not surprisingly, higher APACHE II scores and comorbid malignancy were independently associated with treatment failure. No relationship between ceftaroline MIC or ceftaroline dosing frequency and failure was observed. Although the 600-mg dose of ceftaroline was associated with success in bivariate analyses, it was not associated with success after multivariable regression. However, these findings should be interpreted with great caution. The ceftaroline MIC distribution was narrow (MIC50, 0.5 mg/liter; MIC90, 1 mg/liter), and there was a limited number of isolates with MICs at (21 isolates) or above (3 isolates) the Clinical Laboratory Standards Institute susceptibility breakpoint of 1 mg/liter (31). Part of the rationale for more aggressive every-8-h dosing is to optimize the likelihood of achieving an adequate time that the free-drug concentration exceeds the MIC for isolates at the susceptibility breakpoint (1 mg/liter) (32). Thus, it is possible that there were too few infections truly necessitating more aggressive dosing in this cohort to detect a difference in outcomes across dosing paradigms. It should also be noted that although we attempted to account for patient-specific ceftaroline clearance by stratifying by creatinine clearance, this is merely a crude exploratory analysis. This cannot replace a careful exposure-response analysis that would require pharmacokinetic sampling and known ceftaroline MICs for all patients. As such, the optimal dosing paradigm for ceftaroline remains undefined, but given the observed tolerability of this regimen, it is reasonable to employ every-8-h dosing for difficult-to-treat infections.
There are numerous considerations to bear in mind when interpreting these results. This was a retrospective study, and the findings are subject to the caveats associated with this study design. The primary concern is missing data due to incomplete documentation in the medical record. To address this, we selected objective and easily measureable outcomes, such as all-cause mortality and BSI duration. Study data were limited to inpatient records; thus, efficacy and safety endpoints reflect short-term data. Notably, neutropenia may be underrepresented, considering that this reaction is known to occur with prolonged ceftaroline administration (33). This analysis included three academic medical centers, and it is unclear if these findings apply to other sites with differing patient populations and practice patterns. Ceftaroline was predominantly given as the second or third directed therapy. Given the impact that prior therapy could have on infection and the organism, it is unclear if the observed outcomes, particularly BSI duration post-ceftaroline treatment, would be consistent if ceftaroline was given at the onset of MRSA BSI. Finally, it is important to note that susceptibility testing was not done centrally but at each individual hospital microbiology laboratory. MIC determinations were not done via broth microdilution according to Clinical and Laboratory Standards Institute guidelines, which is used for establishing susceptibility breakpoints.
In conclusion, ceftaroline was well tolerated and demonstrated favorable clinical response rates for MRSA BSI as monotherapy or combination therapy. Clearance of bloodstream infection was rapid following the initiation of ceftaroline treatment in the majority of patients with an active BSI at the start of ceftaroline therapy. This finding, along with previously reported observational data, demonstrates the viability of ceftaroline as a potential treatment option for MRSA BSI. This includes BSI secondary to high-inoculum sources, such as infective endocarditis and pneumonia, and following a perceived failure of prior therapy. With the limited number of cases where ceftaroline was used as the initial antibiotic therapy, its merit as a first-line agent remains unclear. However, these data suggest that ceftaroline is ready for direct comparative study with the current standard of care for MRSA BSI.
MATERIALS AND METHODS
Study design and population.
This was a retrospective, multicenter, observational study conducted between 2011 and 2015 at the Detroit Medical Center, the Henry Ford Health System, and the University of Florida Health-Shands Hospital. Patients aged 18 years and older with at least one positive blood culture for MRSA meeting CDC BSI criteria and receiving at least 72 h of ceftaroline were included (34). This study was approved by the institutional review board at each institution, and a waiver of informed consent was granted.
Patient data elements and collection.
Eligible subjects were identified by screening a list of all patients receiving ceftaroline at each institution during the study period. Patient data were extracted from the medical record by trained reviewers using a structured data collection form within REDCap (Research Electronic Data Capture) (Vanderbilt University), an electronic data capture tool hosted at Wayne State University (35). Data elements included patient demographics, past medical history, comorbid conditions, hospitalizations in the past year, receipt of systemic antibiotics in the past 90 days, surgery, chemotherapy/radiation therapy, or receipt of immunosuppressive medications in the past 30 days. The degree of patient comorbidity was quantified by using the Charlson comorbidity index (36). Severity of illness was quantified by using the APACHE II score, using the worst parameters within 24 h of the index MRSA blood culture (37, 38). The source(s) of MRSA BSI was based on the treating physicians' notes and available clinical and diagnostic data. Microbiological data, including antibiotic susceptibilities determined by MicroScan, Vitek II, BD Phoenix, and/or Etest, were collected from the medical record. Polymicrobial BSI was defined as the isolation of an additional pathogen satisfying CDC BSI criteria within 24 h of the index MRSA BSI isolate (34). Treatment data, including infectious disease team consultation, source control attempt(s), and antimicrobial treatment data including associated laboratory data, were documented. Combination therapy was defined as the receipt of concurrent MRSA-active therapy with ceftaroline for ≥24 h.
Outcomes.
The primary outcome of interest was clinical success defined as BSI clearance, cessation of BSI signs and symptoms (i.e., fever and leukocytosis) by the end of therapy or discharge (whichever was sooner), and the patient being alive at hospital discharge. Patients not classified as having success were considered to have experienced treatment failure. Secondary outcomes included all-cause in-hospital mortality, BSI clearance and duration post-ceftaroline initiation, and hospital length of stay post-ceftaroline initiation. Clearance of bloodstream infection was defined as a series of two consecutive negative blood cultures, with the date of clearance being considered the day of the first negative culture. Safety outcomes included specific, a priori-defined inpatient adverse drug reactions, including rash, Clostridium difficile-associated diarrhea, and neutropenia, after the first dose of ceftaroline. Neutropenia was objectively defined as a decrease in the neutrophil count to <1,500 cells/mm3 or a >50% decrease from the baseline (first ceftaroline dose) if the baseline was ≤1,500 cells/mm3 from the first dose to 72 h after the last ceftaroline dose (39).
Data analysis.
Patients were grouped into two populations for data analysis: a safety population consisting of all included patients and an efficacy population consisting of patients who had not cleared their BSI prior to the receipt of the first ceftaroline dose. Each of these populations was analyzed as a whole and further stratified by whether ceftaroline was received as monotherapy or within a combination regimen. For the purposes of this analysis, combination therapy was defined as the receipt of a concomitant MRSA-active antibiotic(s) for ≥24 h. Descriptive statistics, including baseline and clinical characteristics, and outcomes were generated for each population and subgroup. The time to BSI clearance post-ceftaroline initiation was plotted by using Kaplan-Meier estimation.
Secondary, exploratory analyses were also conducted on the efficacy population to examine factors associated with clinical failure. The relationship between ceftaroline MIC and clinical failure was examined by modeling the ceftaroline MIC as both continuous and ordinal data via the Mann-Whitney U test and chi-squared test/Fisher's exact test, respectively. Clinical success by ceftaroline dosing frequency was examined via Pearson's chi-squared test/Fisher's exact test while stratifying by the FDA-labeled renal dose adjustment categories. Independent predictors of treatment failure were sought through multivariable logistic regression analysis conducted in the efficacy population. Variables were selected for the regression model based on bivariate comparisons between patients experiencing clinical success and those experiencing failure by Pearson's chi-squared test or Fisher's exact test and Student's t test or the Mann-Whitney U test as appropriate. Variables associated with failure at a P value of <0.2 in bivariate analyses with biological plausibility were entered into the logistic regression model simultaneously and removed in a backward, stepwise fashion, being retained in the model if the adjusted P value was <0.1. Model fit was assessed with the Hosmer-Lemeshow goodness-of-fit test; models with a nonsignificant result were considered adequate. Multicolinearity of candidate regression models was assessed via the variance inflation factor, with values of <3 being considered acceptable. All calculations were performed by using SPSS Statistics, IBM SPSS software, version 22.0 (IBM Corp., Armonk, NY), with P values of <0.05 being considered significant.
Supplementary Material
ACKNOWLEDGMENTS
S.L.D. is a grant recipient and has served on the advisory boards of Allergan and Merck & Co. and has served on the advisory board of Melinta. M.J.R. is a grant recipient of, consultant for, member of the advisory board of, and on the speaker's bureau for Allergan, Bayer, Cempra Inc., Merck & Co., The Medicines Company, Sunovian, and Theravance and is supported in part by NIH grants R21 AI109266-01 and R01 AI121400-01. All other authors report no potential conflicts.
The investigators received no funding for the execution of this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02015-16.
REFERENCES
- 1.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S, Emerging Infections Program-Active Bacterial Core Surveillance MRSA Surveillance Investigators. 2013. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr, Hellmich M, Hopkins S, Kern WV, Llewelyn MJ, Rieg S, Rodriguez-Bano J, Scarborough M, Seifert H, Soriano A, Tilley R, Torok ME, Weiss V, Wilson AP, Thwaites GE. 2014. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 68:242–251. doi: 10.1016/j.jinf.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodise TP, McKinnon PS. 2005. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis 52:113–122. doi: 10.1016/j.diagmicrobio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 6.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 7.Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, Laplante KL, Potoski BA, Rybak MJ. 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus bloodstream infection. Antimicrob Agents Chemother 57:4252–4259. doi: 10.1128/AAC.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 9.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. 2012. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis 54:51–58. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 10.Murray KP, Zhao JJ, Davis SL, Kullar R, Kaye KS, Lephart P, Rybak MJ. 2013. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis 56:1562–1569. doi: 10.1093/cid/cit112. [DOI] [PubMed] [Google Scholar]
- 11.Claeys KC, Zasowski EJ, Casapao AM, Lagnf AM, Nagel JL, Nguyen CT, Hallesy JA, Compton MT, Kaye KS, Levine DP, Davis SL, Rybak MJ. 2016. Propensity matched analysis of early daptomycin versus vancomycin for methicillin-resistant S. aureus bloodstream infections: daptomycin improves outcomes regardless of vancomycin MIC. Antimicrob Agents Chemother 60:5841–5848. doi: 10.1128/AAC.00227-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 13.Barber KE, Ireland CE, Bukavyn N, Rybak MJ. 2014. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther 3:35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma M, Riederer K, Chase P, Khatib R. 2008. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 27:433–437. doi: 10.1007/s10096-007-0455-5. [DOI] [PubMed] [Google Scholar]
- 15.Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B, Martin C, Lopez-Medrano F, de Gopegui ER, Blanco JR, Garcia-Pardo G, Calbo E, Montero M, Granados A, Jover A, Duenas C, Pujol M. 2014. Emergence of resistance to daptomycin in a cohort of patients with methicillin-resistant Staphylococcus aureus persistent bacteraemia treated with daptomycin. J Antimicrob Chemother 69:568–571. doi: 10.1093/jac/dkt396. [DOI] [PubMed] [Google Scholar]
- 16.Sader HS, Flamm RK, Jones RN. 2013. Antimicrobial activity of ceftaroline tested against staphylococci with reduced susceptibility to linezolid, daptomycin, or vancomycin from U.S. hospitals, 2008 to 2011. Antimicrob Agents Chemother 57:3178–3181. doi: 10.1128/AAC.00484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casapao AM, Davis SL, Barr VO, Klinker KP, Goff DA, Barber KE, Kaye KS, Mynatt RP, Molloy LM, Pogue JM, Rybak MJ. 2014. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother 58:2541–2546. doi: 10.1128/AAC.02371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho TT, Cadena J, Childs LM, Gonzalez-Velez M, Lewis JS II. 2012. Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother 67:1267–1270. doi: 10.1093/jac/dks006. [DOI] [PubMed] [Google Scholar]
- 19.Lin JC, Aung G, Thomas A, Jahng M, Johns S, Fierer J. 2013. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother 19:42–49. doi: 10.1007/s10156-012-0449-9. [DOI] [PubMed] [Google Scholar]
- 20.Polenakovik HM, Pleiman CM. 2013. Ceftaroline for meticillin-resistant Staphylococcus aureus bacteraemia: case series and review of the literature. Int J Antimicrob Agents 42:450–455. doi: 10.1016/j.ijantimicag.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Tattevin P, Boutoille D, Vitrat V, Van Grunderbeeck N, Revest M, Dupont M, Alfandari S, Stahl JP. 2014. Salvage treatment of methicillin-resistant staphylococcal endocarditis with ceftaroline: a multicentre observational study. J Antimicrob Chemother 69:2010–2013. doi: 10.1093/jac/dku085. [DOI] [PubMed] [Google Scholar]
- 22.Fabre V, Ferrada M, Buckel WR, Avdic E, Cosgrove SE. 2014. Ceftaroline in combination with trimethoprim-sulfamethoxazole for salvage therapy of methicillin-resistant Staphylococcus aureus bacteremia and endocarditis. Open Forum Infect Dis 1:ofu046. doi: 10.1093/ofid/ofu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paladino JA, Jacobs DM, Shields RK, Taylor J, Bader J, Adelman MH, Wilton GJ, Crane JK, Schentag JJ. 2014. Use of ceftaroline after glycopeptide failure to eradicate meticillin-resistant Staphylococcus aureus bacteraemia with elevated vancomycin minimum inhibitory concentrations. Int J Antimicrob Agents 44:557–563. doi: 10.1016/j.ijantimicag.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Sakoulas G, Moise PA, Casapao AM, Nonejuie P, Olson J, Okumura CY, Rybak MJ, Kullar R, Dhand A, Rose WE, Goff DA, Bressler AM, Lee Y, Pogliano J, Johns S, Kaatz GW, Ebright JR, Nizet V. 2014. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 36:1317–1333. doi: 10.1016/j.clinthera.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 25.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. 2015. Outcomes associated with bacteremia in the setting of methicillin-resistant Staphylococcus aureus pneumonia: a retrospective cohort study. Crit Care 19:312. doi: 10.1186/s13054-015-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano A, Martinez JA, Mensa J, Marco F, Almela M, Moreno-Martinez A, Sanchez F, Munoz I, Jimenez de Anta MT, Soriano E. 2000. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis 30:368–373. doi: 10.1086/313650. [DOI] [PubMed] [Google Scholar]
- 27.Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 28.Sakoulas G, Okumura CY, Thienphrapa W, Olson J, Nonejuie P, Dam Q, Dhand A, Pogliano J, Yeaman MR, Hensler ME, Bayer AS, Nizet V. 2014. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 92:139–149. doi: 10.1007/s00109-013-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barber KE, Rybak MJ, Sakoulas G. 2015. Vancomycin plus ceftaroline shows potent in vitro synergy and was successfully utilized to clear persistent daptomycin-non-susceptible MRSA bacteraemia. J Antimicrob Chemother 70:311–313. doi: 10.1093/jac/dku322. [DOI] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2014. M100-S24: performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Canut A, Isla A, Rodriguez-Gascon A. 2015. Pharmacokinetic/pharmacodynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skin-structure infections in patients with normal and impaired renal function. Int J Antimicrob Agents 45:399–405. doi: 10.1016/j.ijantimicag.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 33.LaVie KW, Anderson SW, O'Neal HR, Rice TW, Saavedra TC, O'Neal CS. 2016. Neutropenia associated with long-term ceftaroline use. Antimicrob Agents Chemother 60:264–269. doi: 10.1128/AAC.01471-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Stevens V, Lodise TP, Tsuji B, Stringham M, Butterfield J, Dodds Ashley E, Brown K, Forrest A, Brown J. 2012. The utility of acute physiology and chronic health evaluation II scores for prediction of mortality among intensive care unit (ICU) and non-ICU patients with methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 33:558–564. doi: 10.1086/665731. [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Institute Cancer Therapy Evaluation Program. 1999. Common toxicity criteria manual. Version 2.0. National Cancer Institute, Rockville, MD: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


