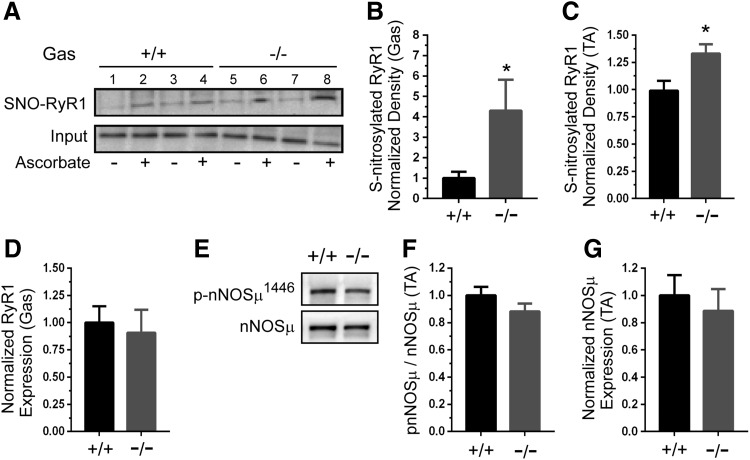
FIG. 3.
GSNOR negatively regulates RyR1 Ca2+ channel S-nitrosylation. Determination of RyR1 S-nitrosylation status using SNO-RAC assay. Ascorbic acid, which converts nitrosothiol groups to thiols, was included as a specificity control. (A) RyR1 S-nitrosylation was increased in GSNOR−/− gastrocnemius. Upper panel: representative Western blot showing two wild-type (+/+) control (lanes 1–4) and two GSNOR−/− gastrocnemius samples (lanes 5–8). Lower panel: representative blot showing RyR1 input. (B) Quantitation revealed a marked approximately four-fold increase in RyR1 S-nitrosylation in the gastrocnemius (gas). (C) RyR1 hypernitrosylation also occurred in GSNOR−/− tibialis anterior muscles. (D) Gastrocnemius RyR1 protein expression was unaffected by loss of GSNOR. (E) Levels of nNOSμ and activated (Ser1446 phosphorylated) nNOSμ were determined. Representative Western blots showing expression of nNOSμ and active ser1446 phosphorylated nNOSμ in WT and GSNOR−/− TA muscle. (F) Quantitative analysis showed that the fraction of active ser1446 phosphorylated nNOSμ was unaffected by GSNOR depletion. (G) Quantitative analysis showed that nNOSμ protein expression was similar between WT and GSNOR−/− mice. A to D, n = 8 and 7, for WT and GSNOR−/− groups, respectively. E to G, n = 6 for all groups. * p < 0.05. SNO-RAC, S-nitrosothiol resin-assisted capture.