Figure 1.
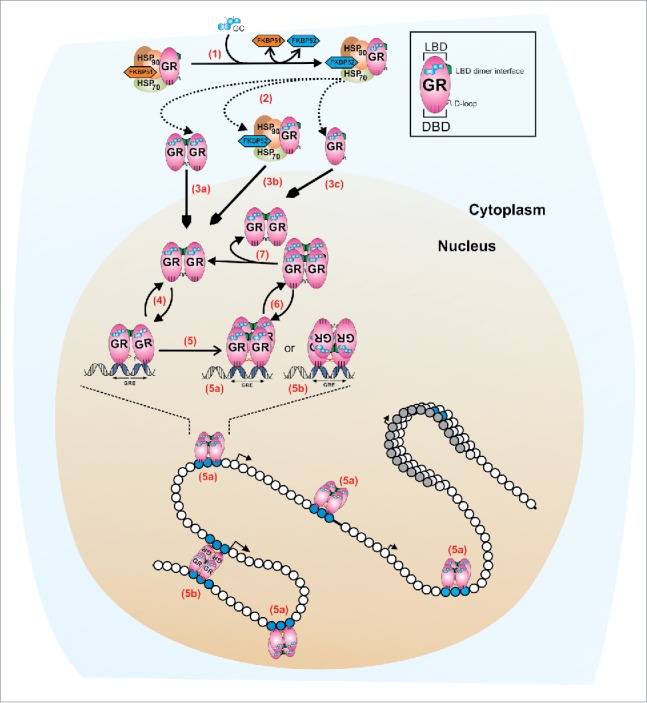
A revised model for GR quaternary structure dynamics. In the absence of ligand, the monomeric cytoplasmic GR forms a heterocomplex with Hsp90, Hsp70, FKBP51, and other proteins (not shown). (1) Ligand binding (GC) induces a conformational change in the GR that leads to either FKBP51-FKBP52 exchange within the heterocomplex, or the complete dissociation from the complex (2). At least two mechanisms appear to regulate the influx of GR molecules to the nuclear compartment: a microtubule/Hsp90 dependent pathway (3b, microtubule not shown) and a microtubule/Hsp90 independent pathway (3a, 3c).35 Early in vivo studies suggests that GR is a dimer before translocation15 (3a) but a monomeric population cannot be rule out (3c). Nucleoplasmic GR appears to be mostly dimeric,3,31,34 with at least two contact surfaces localized in the LBD (shown in green) and the DBD (D-loop, shown in black).31 The interaction with specify response elements (GREs) is very dynamic (4), in the order of seconds.42 DNA induces an allosteric change in the receptor's DBD domain51 (D-loop shown in red) which triggers a conformational change in the LBD, allowing the formation of tetramers, either in a head-to-head (5a) or head-to-tail (5b) configuration.3 GR tetramers also exchange dynamically with DNA (6) and, if it is the tetramer the quaternary structure that detaches from DNA, then the dissociation into dimers should occur at a much faster temporal scale (7), to account for the mostly complete population of dimers observed in the nucleoplasm.3 This highly dynamic regulation occurs in the context of a chromatin landscape. We speculate that a head-to-tail configuration (5b) may assist in bridging different points in the genome, thus favoring a looping mechanism between distant regulatory sites.
