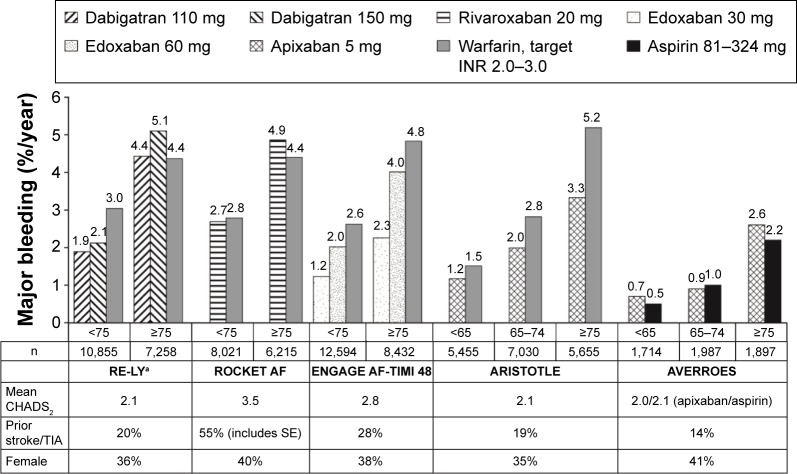
Figure 2.
Rates of major bleeding by age subgroup in Phase III trials of NOACs in patients with NVAF.
Notes: aP<0.05 for interaction between age and treatment. Values represent rates per 100 patient-years. Data from the following studies.27,29,31–33
Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke, TIA, or non-central nervous system thromboembolism doubled; ENGAGE AF-TIMI 48, Evaluation of Efficacy and Safety of Edoxaban versus Warfarin in Subjects with Atrial Fibrillation – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; INR, international normalized ratio; NOAC, non-vitamin K antagonist oral anticoagulant; NVAF, non-valvular atrial fibrillation; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; SE, systemic embolism; TIA, transient ischemic attack.