Figure 2.
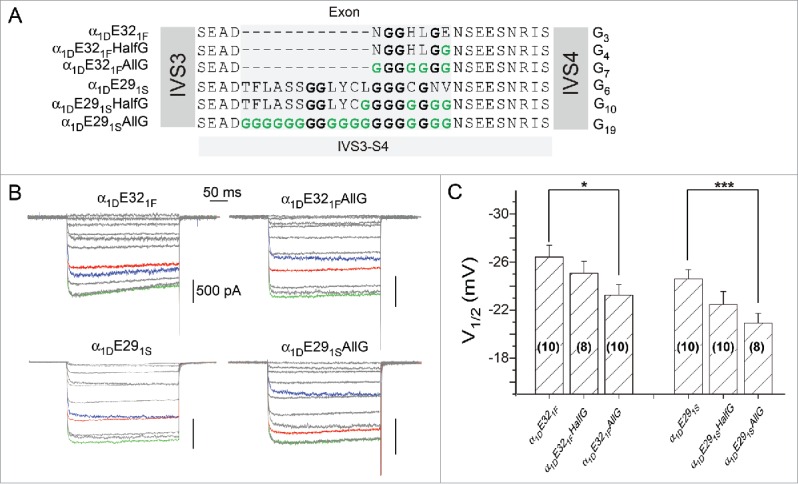
The strategy to change the flexibility of IVS3-S4 linkers by enrichment of glycine residues. (A) The summary of point mutations to glycine residues. Based on chimeric channels of α1DE321F and α1DE291S containing native variants of IVS3-S4, glycine (G) residues gradually replaced the original sites of amino acids for each chimera. According to the percentage of glycine residues, the constructs are named as HalfG or AllG. (B) Exemplar current traces of selected mutant channels in (A), in response to the same voltage protocols and the color scheme as in Figure 1B. Top row and bottom row are based on α1DE321F and α1DE291S, respectively. According to the number of glycine residues (GX), for top row from the left to right, X = 3 or 7; and X = 6 or 19 for the bottom row. The statistics of the group data is shown in (C), with the number of cells indicated for each chimera. *, p < 0.05 and ***, p < 0.001.
