Summary
Background
Observational data have been conflicted regarding the potential role of HIV antiretroviral therapy (ART) as a causative factor for, or protective factor against, COPD. We therefore aimed to investigate the effect of immediate versus deferred ART on decline in lung function in HIV-positive individuals.
Methods
We did a nested substudy within the randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial at 80 sites in multiple settings in 20 high-income and low-to-middle-income countries. Participants were HIV-1 infected individuals aged at least 25 years, naive to ART, with CD4 T-cell counts of more than 500 per µL, not receiving treatment for asthma, and without recent respiratory infections (baseline COPD was not an exclusion criterion). Participants were randomly assigned to receive ART (an approved drug combination derived from US Department of Health and Human Services guidelines) either immediately, or deferred until CD4 T-cell counts decreased to 350 per µL or AIDS developed. The randomisation was determined by participation in the parent START study, and was not specific to the substudy. Because of the nature of our study, site investigators and participants were not masked to the treatment group assignment; however, the assessors who reviewed the outcomes were masked to the treatment group. The primary outcome was the annual rate of decline in lung function, expressed as the FEV1 slope in mL/year; spirometry was done annually during follow-up for up to 5 years. We analysed data on an intention-to-treat basis, and planned separate analyses in smokers and non-smokers because of the known effects of smoking on FEV1 decline. The substudy was registered at ClinicalTrials.gov number NCT01797367.
Findings
Between March 11, 2010, and Aug 23, 2013, we enrolled 1026 participants to our substudy, who were then randomly assigned to either immediate (n=518) or deferred (n=508) ART. Median baseline characteristics included age 36 years (IQR 30–44), CD4 T-cell count 648 per µL (583–767), and HIV plasma viral load 4·2 log10 copies per mL (3·5–4·7). 29% were female and 28% were current smokers. Median follow-up time was 2·0 years (IQR 1·9–3·0). We noted no differences in FEV1 slopes between the immediate and deferred ART groups either in smokers (difference of –3·3 mL/year, 95% CI –38·8 to 32·2; p=0·86) or in non-smokers (difference of –5·6 mL/year, –29·4 to 18·3; p=0·65) or in pooled analyses adjusted for smoking status at each study visit (difference of –5·2 mL/year, –25·1 to 14·6; p=0·61).
Interpretation
The timing of ART initiation has no major short-term effect on rate of lung function decline in HIV-positive individuals who are naive to ART, with CD4 T-cell counts of more than 500 per µL. In light of updated WHO recommendations that all HIV-positive individuals should be treated with ART, regardless of their CD4 T-cell count, our results suggest an absence of significant pulmonary harm with such an approach.
Funding
US National Heart Lung and Blood Institute, US National Institute of Allergy and Infectious Diseases, Division of AIDS, Agence Nationale de Recherches sur le SIDA et les Hipatites Virales (France), Australian National Health and Medical Research Council, Danish National Research Foundation, European AIDS Treatment Network, German Ministry of Education and Research, UK Medical Research Council and National Institute for Health Research, and US Veterans Health Administration Office of Research and Development.
Introduction
Pulmonary complications of HIV-1 infection have been present from the earliest days of the AIDS epidemic, but in the current era of effective combination antiretroviral therapy (ART), such complications have shifted away from opportunistic infections and towards non-infectious complications, especially COPD.1,2 Although the most common cause of COPD is cigarette smoking, findings from observational studies have consistently identified HIV infection as an independent risk factor for COPD, even when adjusted for the high smoking prevalence among HIV-positive individuals.3 Potential mechanisms leading to HIV-associated COPD include respiratory infections, oxidative stress, monocyte activation, T-cell dysregulation, chronic pulmonary inflammation, and changes in the respiratory microbiota.2,4
ART treatment itself has also been implicated as a potential factor in HIV-associated COPD, but the data are conflicting. Findings from two observational studies suggested ART use was associated with an increased risk of COPD,5,6 results from another study showed a lower incidence of COPD among ART users,7 and results from other studies showed no association.8–11 All these studies were limited by their observational designs, in which ART use might be confounded by factors such as socioeconomic status, adherence to medical therapy, and other health behaviours that might affect both the likelihood of ART use and COPD risk.
To address these discrepancies, we did a nested substudy within a randomised controlled trial to test the effect of immediate versus deferred ART on lung function decline in HIV-positive individuals.
Methods
Study design and participants
We did a nested, prospective, controlled, assessor-blinded, substudy within the multisite, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial at 80 sites in multiple settings in 20 high-income and low-to-middle-income countries. The design, methods, and baseline participant characteristics of the parent START trial and this pulmonary substudy have been previously published12–14 and the primary results of the parent START trial have been published15 (for the complete protocol, see appendix). All site institutional review boards or ethics committees approved the substudy.
Briefly, the parent START trial enrolled HIV-1-infected, ART-naive adults aged more than 18 years with CD4 T-cell counts of more than 500 per µL. In addition to the entry criteria for the parent START trial, our additional substudy criteria included the requirement that participants be aged at least 25 years, the age at which lung function begins to decline in most adults. Exclusion criteria were: 1) an episode of respiratory illness within the 6 weeks before baseline spirometry; 2) use of asthma medications for 2 or more consecutive weeks within the 6 months before baseline spirometry; 3) relative contraindications to spirometry, such as chest, abdominal, or eye surgery within the 3 months before baseline spirometry, or known retinal detachment at the time of baseline spirometry; 4) known allergy to albuterol/salbutamol; 5) relative contraindications to albuterol or salbutamol, such as a resting heart rate of more than 110 beats per minute, or a known serious, recurrent or uncontrolled cardiac condition (such as unstable coronary artery disease, decompensated heart failure, or recurrent tachyarrhythmias). Baseline COPD was not an exclusion criterion. We set no limits on enrolment by site, region, sex, or any other factors; all sites had their own methods of recruiting participants. All START substudy participants provided written informed consent specific to their participation in the substudy.
Randomisation and masking
After enrolment and baseline data collection, participants were randomly allocated to an ART treatment strategy of either immediate initiation of ART or deferred initiation until the CD4 T-cell count decreased to 350 per µL or AIDS developed. The randomisation was determined by participation in the parent START study, and was not specific to the substudy. The study protocol required the use of an approved drug combination derived from the guidelines of the US Department of Health and Human Services as the first ART regimen in both study groups.
Randomisation was stratified by clinical site and permuted blocks of different sizes were used to generate the randomisation schedules. Assignments were obtained from a web-based program that verified participant eligibility. Randomisation schedules were generated only for the parent trial; there was no stratification by substudy participation. Because of the nature of the study as a pragmatic trial to compare HIV treatment strategies rather than specific drugs, site investigators and parti cipants were not masked to the treatment group assignment (in either the parent START trial and this substudy). However, outcomes including lung function test results were reviewed by assessors centrally, who were masked to treatment group and reviewed reports with no indicators regarding ART use or randomised treatment assignment.
Procedures
Before randomisation, study participants did post-bronchodilator spirometry using the EasyOne ultrasonic flow device (ndd Medical, Zurich, Switzerland) after inhalation of 180 µg of albuterol or salbutamol via a metered dose inhaler. During the study, spirometry was done once per year during follow-up for up to 5 years. One of the investigators (KMK) centrally reviewed all spirometry test results. Repeat testing was requested when test results did not meet published quality standards.16 We used Global Lung Function Initiative 2012 normative equations17 to determine predicted FEV1, predicted forced vital capacity (FVC), and the lower limit of normal (5th percentile) of the FEV1:FVC ratio.
We assessed current and former cigarette use and defined current smokers as having smoked more than ten cigarettes in the past 30 days. We defined lifelong non-smokers as having reported smoking fewer than 120 cigarettes in their lifetime. We calculated smoking pack-years as (average self-reported number of cigarettes smoked per day/20) × number of years smoked.
We administered the St George’s Respiratory Questionnaire for COPD (SGRQ-C). The SGRQ-C is a validated, participant-completed, 40-item measure of respiratory health status,18 scored from 0 to 100 points, for which higher scores reflect worse respiratory health status. SGRQ-C questionnaires were scored according to the standardised SGRQ-C algorithm, which converts SGRQ-C responses to equivalent scores on the 50-item full version of the SGRQ. The minimal clinically important difference in SGRQ scores is 4 points.19 Certified SGRQ-C language or dialect translations were available for most countries, but not all. In Israel, Thailand, and Peru, the full 50-item version of the SGRQ had been translated and certified for use in these countries, but not the SGRQ-C. Therefore in these countries, we shortened the SGRQ to the SGRQ-C and translations and back-translations were done before site opening. In Cape Town, South Africa, we did new translations and back-translations for Xhosa and Zulu from the original English version of the SGRQ-C. For sites in several countries (India, Nigeria, and Uganda, representing 18% of study participants), there were too many dialects to do timely translations for all participants, so the SGRQ-C was not administered at these sites. We assessed use of respiratory medications (eg, inhalers, oral leukotriene inhibitors, or theophylline) and bronchitis events by self-report annually.
Outcomes
Our primary outcome was rate of lung function decline, expressed as FEV1 slope in mL/year. Secondary outcomes were the 12-month change in SGRQ scores, use of respiratory medications, and incidence of respiratory illnesses (ie, bronchitis events).
Statistical analysis
Because of the known effects of smoking on FEV1 slope, we planned analyses in baseline smokers and non-smokers separately. We projected that sample size targets of 400 smokers and 600 non-smokers would have 85% power to detect a difference in FEV1 slope of 15·5 mL/year in non-smokers and 19·0 mL/year in smokers.14 Data from the Lung Health Study20 showed that with smoking cessation, FEV1 slope tends to be similar to that in non-smokers. Therefore, we analysed former smokers with the non-smoker group in the primary analyses.
The START study’s data and safety monitoring board recommended early termination of the parent trial at its May 15, 2015, meeting, because the study showed clear reductions in AIDS events and non-AIDS events in the immediate ART arm. Results were announced by the US National Institutes of Health on May 27, 2015. Therefore, follow-up data were censored on the last day of masked allocation—May 26, 2015,—or the date of last study contact.
For our primary outcome of FEV1 slope, we used repeated-easures mixed models, with random withinperson intercept and slope, with all available spirometry data on a strict intention-to-treat basis by assignment to immediate or deferred ART (see appendix for additional details, including statistical codes). In the case of low-quality spirometry that required repeat testing, we used the most recent test within each study visit window. We tested the linearity of the FEV1 slopes by adding a time-quadratic term to the primary outcome models separately in smokers and non-smokers; the coefficients for the quadratic terms were non-significant (p>0·10) in both smokers and non-smokers and therefore were not included in the final models.
We also did the following secondary analyses: 1) pooling data from baseline smokers and non-smokers with adjustment for smoking status at baseline; 2) pooling data from baseline smokers and non-smokers with adjustment for smoking status at each study visit; 3) restricting analyses to only spirometry tests meeting acceptable quality standards after central review; and 4) censoring spirometry data after ART initiation in the deferred ART arm. Intention-to-treat analyses might not be the most appropriate method for estimating harm, so to address the potential for ART to worsen lung function decline, we also did a secondary analysis of time on ART (allowing each individual to contribute data both on and off ART), and FEV1 slope, regardless of treatment assignment. We assessed heterogeneity by high-income versus low-to-middle-income settings by testing for an interaction between treatment assignment and high-income country (in the regions of USA, Europe, Israel, and Australia) and low-to-middle-income country (in the regions of Africa, India, Latin America, and Thailand).
For the secondary outcome of SGRQ change, we compared the difference between the randomised arms in SGRQ change between baseline and month 12 separately for smokers and non-smokers. We defined incident bronchitis events as those associated with two or more symptoms of cough, wheezing, breathlessness, or increase in sputum production. We calculated the odds of reporting bronchitis events since the previous study visit over follow-up. For the use of respiratory medications, we calculated the odds of reporting respiratory medication use since the previous study visit over follow-up.
We did all statistical analyses with SAS 9.4 (Cary, NC, USA). We registered the substudy at ClinicalTrials.gov (NCT01797367).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
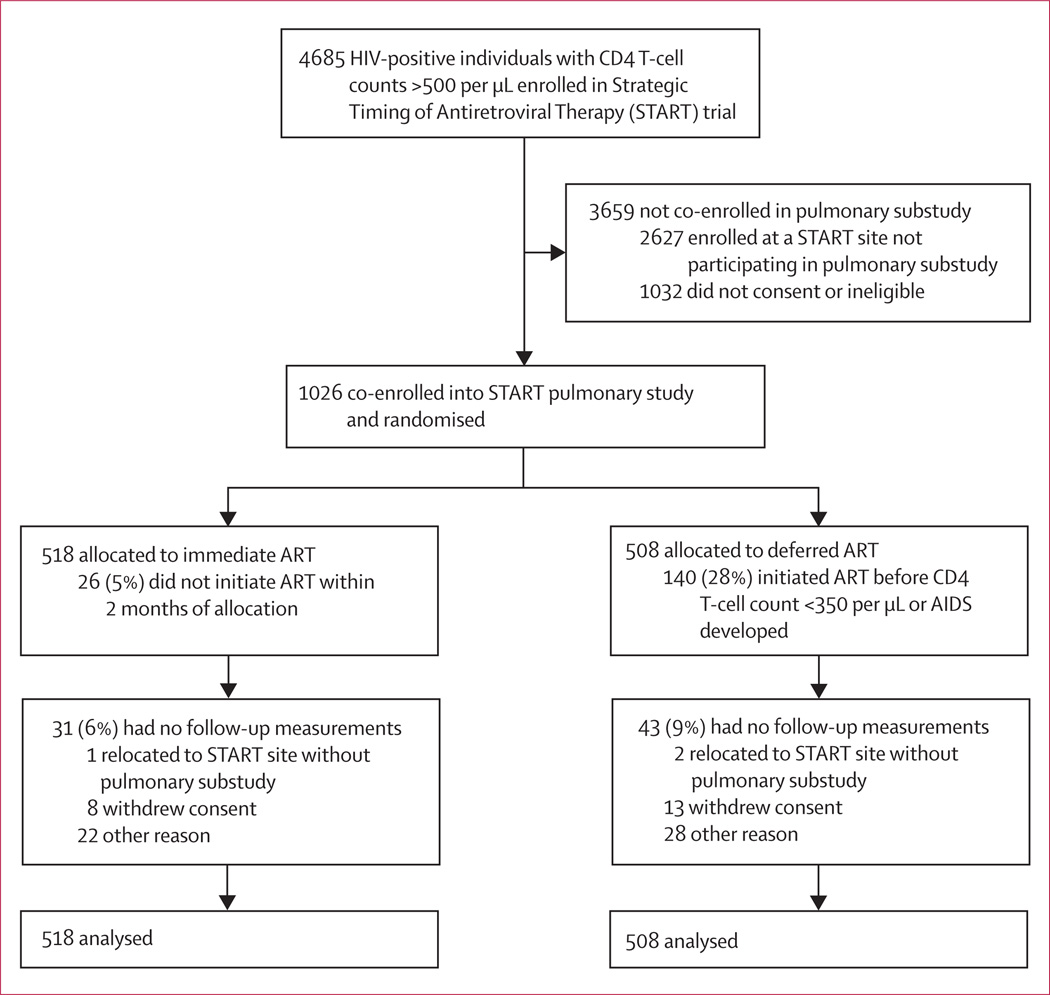
Between March 11, 2010, and Aug 23, 2013, we enrolled 1026 participants in the pulmonary substudy. Participants were randomly assigned to either immediate ART (n=518) or deferred ART (n=508; figure 1). Baseline demo graphic and clinical characteristics were well balanced between the two groups (table 1). The region with highest enrolment was Africa, followed by Europe/ Israel/Australia, Latin America, Asia, and the USA. Overall, the cohort was young (median age 36 years [IQR 30–44]), with a recent HIV diagnosis (median time since diagnosis 1·2 years [0·4–3·5]), median baseline CD4 T-cell count 648 per µL (583–767), median HIV plasma viral load of 4·2 log10 copies per mL (3·5–4·7), and 9% had a baseline HIV plasma viral load less than 400 copies per mL despite reporting being ART naive. 29% were female and most study participants reported sexual HIV acquisition (49% men who have sex with men, 44% heterosexual intercourse).
Figure 1.
Trial profile
Table 1.
Baseline characteristics of the intention-to-treat population
| Immediate ART (n=518) |
Deferred ART (n=508) |
Total (n=1026) |
|
|---|---|---|---|
| Age (years) | 37 (30–44) | 36 (31–43) | 36 (30–44) |
| Female | 145 (28%) | 154 (30%) | 299 (29%) |
| Race | |||
| Black | 198 (38%) | 189 (37%) | 387 (38%) |
| Latino or Hispanic | 93 (18%) | 86 (17%) | 179 (17%) |
| Asian | 52 (10%) | 53 (10%) | 105 (10%) |
| White | 169 (33%) | 175 (34%) | 344 (34%) |
| Other | 6 (1%) | 5 (1%) | 11 (1%) |
| Region | |||
| Africa | 168 (32%) | 160 (31%) | 328 (32%) |
| Asia | 52 (10%) | 51 (10%) | 103 (10%) |
| Europe, Israel, or Australia |
162 (31%) | 151 (30%) | 313 (31%) |
| Latin America | 91 (18%) | 100 (20%) | 191 (19%) |
| USA | 45 (9%) | 46 (9%) | 91 (9%) |
| Likely method of HIV infection | |||
| Injection-drug use | 9 (2%) | 5 (1%) | 14 (1%) |
| Male sexual contact with person of same sex |
258 (50%) | 247 (49%) | 505 (49%) |
| Sexual contact with Person of opposite sex |
225 (43%) | 225 (44%) | 450 (44%) |
| Other or unknown | 26 (5%) | 31 (6%) | 57 (6%) |
| Years HIV positive | 1·1 (0·4–3·5) |
1·2 (0·4–3·6) |
1·2 (0·4–3·5) |
| Laboratory results | |||
| CD4 T-cell count (cells per µL) |
650 (587–768) |
647 (576–765) |
648 (583–767) |
| CD8T–cell count (cells per µL) |
1026 (757–1381) |
1008 (759–1420) |
1019 (758–1394) |
| CD4:CD8 ratio | 0·67 (0·48–0·97) |
0·66 (0·48–0·91) |
0·66 (0·48–0·94) |
| Nadir CD4 T-cell count (cells per µL) |
545 (480–665) |
562 (490–651) |
552 (486–661) |
| Log HIV-RNA (copies per mL) |
4·2 (3·5–4·7) |
4·2 (3·4–4·7) |
4·2 (3·5–4·7) |
| HIV-RNA ≤400 copies per mL |
45 (9%) | 52 (10%) | 97 (9%) |
| Medical history | |||
| Body-mass index (kg/m²) |
24·8 (22·2–28·5) |
24·8 (22·2–28·0) |
24·8 (22·2–28·4) |
| Current smoker | 135 (26%) | 155 (31%) | 290 (28%) |
| Previous smoker | 58 (11%) | 53 (10%) | 111 (11%) |
| Never smoker | 325 (63%) | 300 (59%) | 625 (61%) |
| Pack years smoking (current and previous smokers) |
7·5 (2·5–15·0) |
5·3 (1·7–14·3) |
6·0 (2·0–15·0) |
| Previous cardiovascular disease |
2 (<1%) | 5 (1%) | 7 (1%) |
| Hypertension | 98 (19%) | 94 (19%) | 192 (19%) |
| Diabetes | 22 (4%) | 15 (3%) | 37 (4%) |
| Hepatitis B or C | 39 (8%) | 33 (6%) | 72 (7%) |
| FEV1 (L) | 3·38 (2·62–3·97) |
3·37 (2·69–4·01) |
3·38 (2·67–4·00) |
| FVC (L) | 4·10 (3·28–4·92) |
4·10 (3·27–4·91) |
4·10 (3·28–4·91) |
| FEV1:FVC ratio* | 0·83 (0·78–0·86) |
0·83 (0·78–0·87) |
0·83 (0·78–0·86) |
| <0·7 | 30 (6%) | 24 (5%) | 54 (5%) |
| <lower limit of normal† |
40 (8%) | 27 (5%) | 67 (7%) |
| FEV1 as percentage of predicted*‡ |
95·2 (85·0–104·2) |
97·0 (86·2–103·2) |
96·2 (85·4–103·9) |
| ≥80% | 421 (85%) | 431 (87%) | 852 (86%) |
| 50%-79% | 74 (15%) | 58 (12%) | 132 (13%) |
| 30%-49% | 2 (<1%) | 4 (<1%) | 6 (1%) |
| <30% | 0 | 0 | 0 |
| Respiratory health status (St George’s Respiratory Questionnaire score) | |||
| Total | 7·2 (4·1–13·6) |
7·2 (4·2–13·2) |
7·2 (4·2–13·4) |
| Symptoms | 13·5 (0·9–27·0) |
12·8 (0·9–26·6) |
13·0 (0·9–26·6) |
| Activity | 7·0 (7·0–20·4) |
7·0 (7·0–20·1) |
7·0 (7·0–20·1) |
| Impacts | 2·2 (2·2–6·8) |
2·2 (2·2–6·9) |
2·2 (2·2–6·8) |
Data are median (IQR) or n (%). ART=antiretroviral therapy. FVC=forced vital capacity.
Restricted to spirometry that met quality standards after central quality review (n=497 in the immediate arm; n=493 in the deferred arm).
<5th percentile as predicted by Global Lung Function Initiative equations.
Predicted values from Global Lung Function Initiative equations.
Current smokers comprised 28% of the cohort at baseline, and former and lifelong non-smokers comprised 72%. Of the baseline smokers, 8% quit smoking (reported not smoking at all follow-up visits) and 18% were intermittent smokers (reported smoking at one or more follow-up visits and not smoking at one or more follow-up visits) during follow-up. Of those not smoking at baseline (never smokers or former smokers), 6% reported smoking at one or more follow-up visits. Baseline lung function was generally good (median FEV1 96·2% of predicted [85·4–103·9]) with COPD prevalence at study entry of 6·8%, as previously reported.14
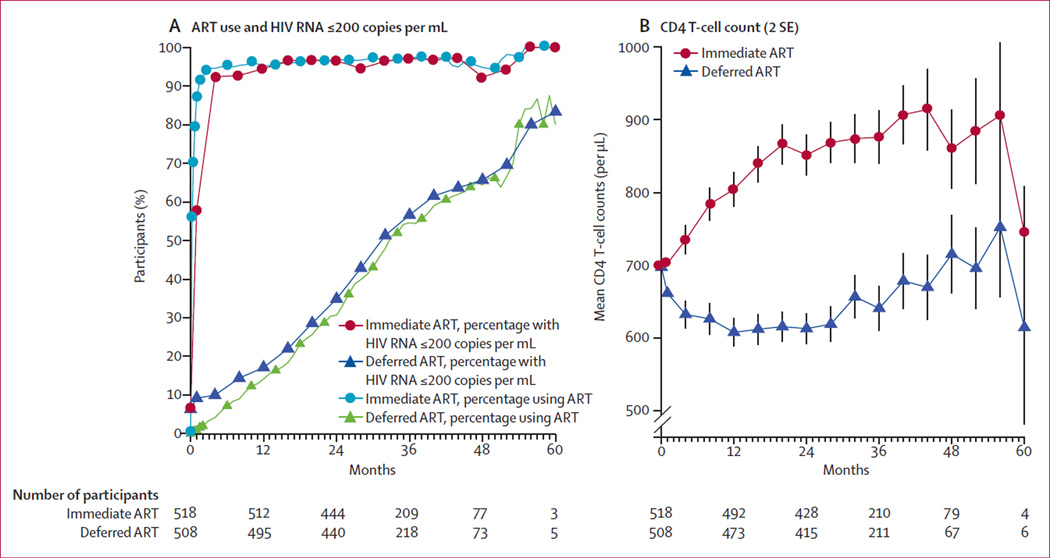
As of the unblinding date (May 26, 2015), ART had been initiated in 99% of the immediate ART arm and 45% in the deferred arm. In the immediate ART arm, mean CD4 T-cell count at the time of ART initiation was 691 per µL (SD 176), mean HIV viral load at ART initiation was 4·1 log10 copies per mL (SD 0·9), and 95·0% of follow-up time was spent on ART (there was a time lag between baseline assessments and initiation of ART; figure 2). In the deferred ART arm, mean CD4 T-cell count at the time of ART initiation was 478 per µL (SD 210), mean HIV viral load at ART initiation was 4·5 log10 copies per mL (SD 0·9), and 26·3% of follow-up time was spent on ART. The percentage of participants with HIV viral suppression closely mirrored ART use. Average CD4 T-cell counts increased markedly during the first year after randomisation in the immediate ART arm and continued to gradually increase thereafter. Conversely, in the deferred ART arm, average CD4 T-cell counts decreased during the first year, then stabilised and subsequently increased slightly as more participants initiated ART (figure 2).
Figure 2. ART use, HIV RNA, and CD4 T-cell counts.
Differences between randomised treatment arms in frequency of antiretroviral therapy (ART) use (A, blue circles and green triangles), HIV RNA of 200 copies per mL or less (A, red circles and blue triangles), and mean CD4 T-cell counts (B), as a function of follow-up time in study.
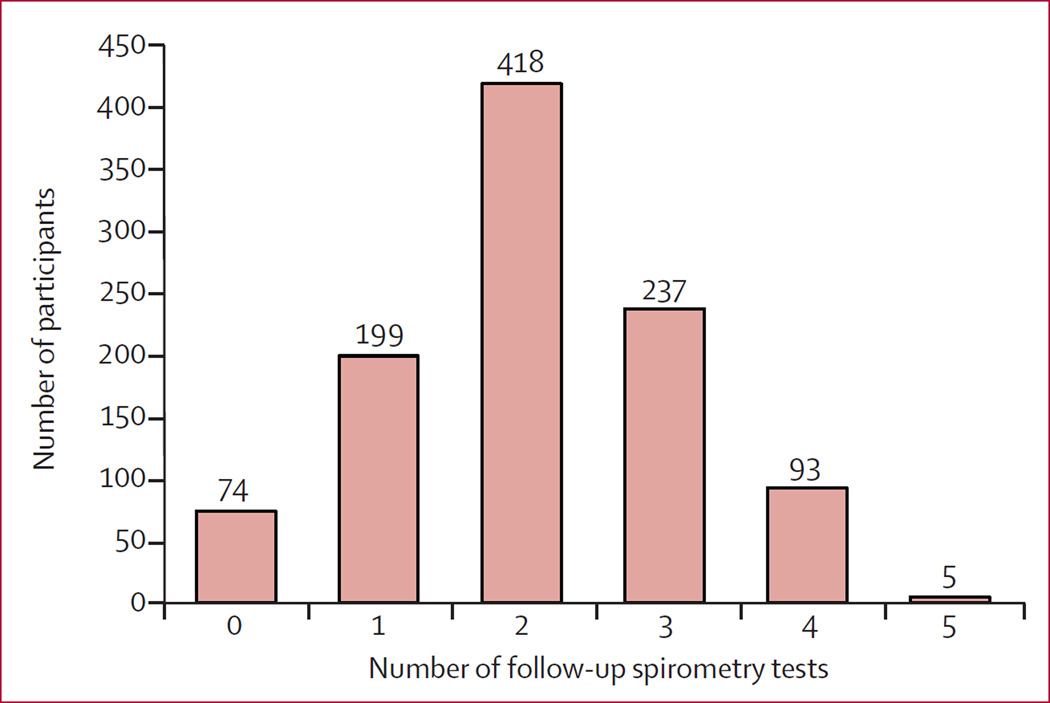
We collected 2143 follow-up spirometry tests over a median follow-up time of 2·0 years (IQR 1·9–3·0). Repeat testing for low-quality spirometry was requested for 131 (6·1%) tests, and ultimately 2039 (95·1%) tests met acceptable quality standards. The proportion of acceptable tests did not differ between the immediate and deferred ART arms (p=0·64). As of the unblinding date, 74 (7%) of those randomly assigned had not completed a follow-up spirometry test (31 [6%] assigned to immediate ART and 43 [9%] assigned to deferred ART; figure 3), but all participants were included in the primary analyses.
Figure 3.
Distribution of number of follow-up spirometry tests collected
For the primary outcome of FEV1 slope, we noted no differences in slopes between the immediate and deferred ART arms either in smokers (difference of –3·3 mL/year, 95% CI –38·8 to 32·2; p=0·86) or in non-smokers (difference of –5·6 mL/year, –29·4 to 18·3; p=0·65; table 2). Analyses pooling baseline smokers and non-smokers, then adjusting for either baseline smoking status or smoking status at each study visit, similarly showed no differences in FEV1 slopes between immediate and deferred ART arms.
Table 2.
Primary outcome of FEV1 slope comparisons
| FEV1 slope (95% CI), mL/year | p value | |
|---|---|---|
| Baseline smokers | ||
| Immediate ART (n=135) | −32·9 (−58·5 to −7·4) | ·· |
| Deferred ART (n=155) | −29·7 (−54·3 to −5·0) | ·· |
| Difference | −3·3 (−38·8 to 32·2) | 0·86 |
| Baseline non-smokers | ||
| Immediate ART (n=383) | −27·8 (–44·2 to –11·4) | ·· |
| Deferred ART (n=353) | −22·2 (−39·6 to −4·9) | ·· |
| Difference | −5·6 (−29·4 to 18·3) | 0·65 |
| Pooled analysis adjusted for baseline smoking status | ||
| Immediate ART (n=518) | −29·1 (−42·9 to −15·4) | ·· |
| Deferred ART (n=508) | −24·5 (−38·6 to −10·3) | ·· |
| Difference | −4·7 (−24·4 to 15·1) | 0·64 |
| Pooled analysis adjusted for smoking status at each study visit | ||
| Immediate ART (n=518) | −28·8 (−42·6 to −14·9) | ·· |
| Deferred ART (n=508) | −23·6 (−37·8 to −9·3) | ·· |
| Difference | −5·2 (−25·1 to 14·6) | 0·61 |
Data are from groups of patients randomly assigned to either immediate or deferred ART initiation. ART=antiretroviral therapy.
Secondary analyses showed no difference in FEV1 slopes between the two arms when 1) restricting to only spirometry meeting acceptable quality standards, 2) censoring deferred arm data at the time of ART initiation, or 3) restricting to both (appendix). Our secondary safety analysis of time on ART and FEV1 slope, regardless of treatment assignment, also showed no relationship between time on ART and FEV1 slope in smokers (p=0·24) or non-smokers (p=0·37). We noted no interaction between the effects of immediate versus deferred ART on FEV1 slope in high-income versus low-to-middle-income countries in smokers (p=0·65) or non-smokers (p=0·71).
We collected 716 SGRQ-C questionnaires at baseline and month 12, of which 677 had complete information to calculate total SGRQ scores at both timepoints. The proportion of participants missing SGRQ-C data did not differ between the immediate and deferred ART arms (13% vs 16%, respectively; p=0·28). We noted no difference in total SGRQ score change between the two treatment arms in smokers or in non-smokers (table 3). There were also no differences between the two treatment arms in any of the individual SGRQ domains for either smokers or non-smokers, with the exception that the symptoms domain score in smokers favoured the immediate ART arm.
Table 3.
Changes in St George’s Respiratory Questionnaire (SGRQ) scores
| Immediate ART |
Deferred ART |
Difference in mean change from baseline* |
p value | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline* | Month 12* | Change from baseline† |
n | Baseline* | Month 12* | Change from baseline† |
||||
| Smokers | |||||||||||
| Total | 102 | 11·7 (94) | 10·6 (10·0) | −1·1 (11·1) | 119 | 12·8 (9·9) | 12·3 (10·4) | −0·5 (7·2) | −1·1 (1·1) | 0·34 | −3·3 to 1·1 |
| Symptoms | 111 | 20·8 (15·4) | 17·9 (14·8) | −2·9 (15·6) | 122 | 21·5 (16·5) | 23·1 (18·8) | 1·6 (15·8) | −4·8 (1·9) | 0·01 | −8·5 to −1·1 |
| Activity | 102 | 16·7 (14·5) | 14·6 (14·3) | −2·0 (14·5) | 119 | 17·5 (14·4) | 17·1 (16·0) | −0·3 (11·6) | −2·0 (1·6) | 0·23 | −5·2 to 1·2 |
| Impacts | 103 | 6·5 (8·5) | 6·4 (9·0) | −0·1 (11·4) | 119 | 8·0 (8·9) | 6·7 (8·5) | −1·2 (6·7) | 0·3 (1·1) | 0·76 | −1·8 to 2·4 |
| Non-smokers | |||||||||||
| Total | 241 | 9·8 (8·9) | 8·7 (8·6) | −1·1 (8·4) | 215 | 9·6 (8·9) | 10·0 (11·3) | 0·4 (10·5) | −1·4 (0·8) | 0·08 | −3·0 to 0·2 |
| Symptoms | 253 | 15·0 (14·0) | 13·2 (15·2) | −1·9 (14·7) | 226 | 14·1 (12·6) | 14·5 (15·6) | 0·4 (14·9) | −1·9 (1·2) | 0·13 | −4·3 to 0·5 |
| Activity | 246 | 14·8 (13·5) | 13·5 (13·4) | −1·3 (12·4) | 222 | 13·9 (13·7) | 14·6 (15·9) | 0·7 (15·2) | −1·6 (1·2) | 0·17 | −3·9 to 0·7 |
| Impacts | 248 | 6·4 (8·4) | 5·7 (8·2) | −0·7 (9·5) | 218 | 6·2 (8·2) | 6·4 (9·8) | 0·2 (10·4) | −0·8 (0·8) | 0·33 | −2·3 to 0·8 |
SGRQ scores were measured in participants randomly assigned to immediate versus deferred antiretroviral therapy (ART) initiation. Higher SGRQ scores indicate worse respiratory health status. The minimal clinically important difference in the total SGRQ score is 4 points; minimal clinically important differences for the symptoms, activity, and impact domains are not established.
Data are means (SD).
Data are means (SE) adjusted for baseline value.
We showed no difference in the risk of developing self-reported episodes of bronchitis between the immediate and deferred ART arms in smokers or in non-smokers (table 4). We also noted no difference in the odds of self-reported respiratory medication use between the immediate and deferred ART arms in smokers or in non-smokers (table 5).
Table 4.
Effect of immediate versus deferred antiretroviral therapy (ART) initiation on bronchitis episodes
| Immediate ART |
Deferred ART |
p value* | |||
|---|---|---|---|---|---|
| n | Number (%) who had a bronchitis event |
n | Number (%) who had a bronchitis event |
||
| Smokers | |||||
| Baseline | 135 | 49 (36%) | 155 | 54 (35%) | |
| Year 1 | 117 | 34 (29%) | 126 | 37 (29%) | 1·00 |
| Year 2 | 96 | 27 (28%) | 110 | 24 (22%) | 0·33 |
| Year 3 | 58 | 11 (19%) | 65 | 10 (15%) | 0·64 |
| Year 4 | 24 | 8 (33%) | 22 | 2 (9%) | 0·07 |
| Non-smokers | |||||
| Baseline | 383 | 96 (25%) | 353 | 76 (22%) | |
| Year 1 | 339 | 62 (18%) | 302 | 55 (18%) | 1·00 |
| Year 2 | 295 | 45 (15%) | 265 | 37 (14%) | 072 |
| Year 3 | 130 | 20 (15%) | 120 | 17 (14%) | 0·86 |
| Year 4 | 42 | 11 (26%) | 29 | 4 (14%) | 025 |
Effects grouped by treatment and smoking status. Odds ratio from Generalised Estimating Equation with outcome as having any episodes of bronchitis at each follow-up visit: for smokers 1·16 (95% CI 0·66–2·02, p=0·61; for non-smokers 0·89 (0·60–1·32), p=0·57
From Fisher’s exact test.
Table 5.
Effect of immediate versus deferred antiretroviral therapy (ART) initiation on self-reported respiratory medication use
| Immediate ART |
Deferred ART |
p value* | |||
|---|---|---|---|---|---|
| n | Number (%) who reported respiratory medication use |
n | Number (%) who reported respiratory medication use |
||
| Smokers | |||||
| Year 1 | 117 | 3 (3%) | 126 | 3 (2%) | 1·00 |
| Year 2 | 91 | 1 (1%) | 99 | 4 (4%) | 037 |
| Year 3 | 54 | 1 (2%) | 58 | 2 (3%) | 1·00 |
| Year 4 | 21 | 0 | 21 | 1 (5%) | 1·00 |
| Non-smokers | |||||
| Year 1 | 339 | 8 (2%) | 302 | 7 (2%) | 1·00 |
| Year 2 | 277 | 4 (1%) | 242 | 4 (2%) | 1·00 |
| Year 3 | 120 | 3 (3%) | 107 | 4 (4%) | 071 |
| Year 4 | 38 | 0 | 26 | 2 (8%) | 0·16 |
Effects grouped by treatment and smoking status. Odds ratio from Generalised Estimating Equation with outcome as having used respiratory medications since the last study visit: for smokers 046 (95% CI 0·15–146), p=0·19; for non-smokers 056 (0·24–131), 0·18.
From Fisher’s exact test.
Discussion
Over a median follow-up of 2 years, the results from our large, multicentre, international randomised trial showed that among ART-naive, HIV-positive individuals with CD4 T-cell counts of more than 500 per µL, the timing of ART initiation had no effect on the rate of lung function decline, for immediate ART compared with deferral of ART until CD4 cell counts were less than 350 per µL. In light of the results from other randomised trials and international HIV treatment guidelines15,21,22 that support immediate initiation of ART in all HIV-positive individuals regardless of CD4 T-cell count, our data suggest no major benefit or harm to lung function associated with such an approach, although we note that our wide CIs cannot fully exclude either benefit or harm.
Concerns about pulmonary harms from ART were based on findings from two single-centre, cross-sectional studies.5,6 In one of these studies (n=215), a lower FEV1:FVC ratio was independently associated with increasing age, more pack-years of smoking, a history of bacterial pneumonia, and use of ART.5 Findings from the other study (n=167) similarly showed that COPD was independently associated with more pack-years of smoking, a history of intravenous drug use, and ART use (odds ratio 6·22, 95% CI 1·19–32·43).6 Such potential pulmonary harms of ART were postulated to potentially be related to an immune reconstitution inflammatory syndrome-like phenomenon.
By contrast with these two cross-sectional studies, results from two other prospective, observational studies suggested that ART might reduce COPD risk in HIV-positive individuals. From administrative data in the US Veterans Aging Cohort Study,7 ART use was associated with a lower incidence of COPD (incidence rate ratio [IRR] 0·90, 95% CI 0·82–0·99), but when the results were adjusted for smoking the association was no longer significant (IRR 0·93, 0·73–1·18). In the AIDS Linked to the Intravenous Experience cohort,9 individuals with higher HIV RNA had a faster decrease in FEV1. However, ART use itself was not associated with a difference in FEV1 slope. The findings from our randomised trial suggest that the discrepant associations between ART use and lung function decline in observational studies might result from confounding inherent in observational study designs, where ART use might be associated with other factors related to lung function decline such as access to care, self-efficacy and other confounders, both currently known and unknown.
Our trial was restricted to participants with initially high CD4 T-cell counts (>500 per µL) and even in the deferred ART arm, average CD4 T-cell counts were more than 600 per µL during follow-up. Therefore, our results cannot be extrapolated to patients who present with, or develop, lower CD4 T-cell counts. Findings from several observational studies suggest that low CD4 T-cell counts are associated with higher COPD risk,9,23 but low CD4 T-cell counts in observational studies, like ART use, might be associated with other confounders. We also excluded individuals receiving treatment for asthma and those with recent respiratory infection symptoms, so our results are not generalisable to such patients who might be newly initiating ART.
We also noted no differences in respiratory health status, as assessed by the total SGRQ score, between the two ART treatment strategies in either smokers or non-smokers. We noted a difference favouring immediate ART for the SGRQ symptoms domain in smokers, but given the multiple comparisons, the relevance of this finding is unclear. The minimal clinically important difference in the SGRQ symptoms domain is also not established. We also note that in this trial, participants were not masked to treatment assignment, and therefore self-reported measures such as SGRQ and bronchitis events might have been subjected to some degree of bias. The SGRQ was not administered in India, Nigeria, and Uganda, so our SGRQ results do not reflect respiratory health status in those countries.
Results from some studies have suggested a high prevalence of respiratory symptoms in HIV-positive individuals. In a study of 199 HIV-positive men in Canada, the median SGRQ score was 32·0 points (of a maximum of 100 points), which is worse than our median SGRQ score of 7·2 points.24 This difference might reflect our cohort’s young age and recent diagnosis of HIV infection compared with the Canadian cohort, in which the mean age was 49 years and years of known HIV diagnosis was 12 years. The fairly good baseline respiratory health status of our study participants might have restricted our ability to detect significant differences in respiratory health status between the ART strategies.
Our study had several strengths. The large sample size and randomised design to immediate vs deferred ART is unique and optimised the ability to balance confounders, both currently known and currently unknown. The randomisation to deferred ART will likely never be repeated again in the future, because of findings from the parent START trial that showed that all HIV-positive individuals should be immediately treated with ART. Our international trial enrolled participants from high-income, middle-income, and low-income countries and therefore our findings have broad generalisability to HIV-positive individuals in widely differing HIV treatment settings.
Our study also had several limitations. We measured only post-bronchodilator spirometry, and did not include more time-consuming or sophisticated measures such as prebronchodilator spirometry, single-breath diffusing capacity of carbon monoxide, or quantitative CT of the thorax. We chose to not collect these other measures, partly because of the costs and logistical challenges of an already-complex parent study, and difficulties in implementing and standardising such measures in our 80-site, 20-country trial in both high-income and low-to-middle-income countries. We note that post-broncho dilator FEV1 is currently the gold standard for assessing lung function loss and diagnosing COPD, which was the primary focus of our study.
Another limitation of our trial was the short follow-up time. Because of the early termination of the parent trial, we had a short median spirometry follow-up time of 2 years. Other studies assessing FEV1 slope as a primary outcome have often had longer follow-up times, such as the 4-year UPLIFT trial of tiotropium25 and the 5-year Lung Health Study of smoking cessation.26 The SUMMIT trial of inhalers,27 where FEV1 slope was a secondary outcome, had a median follow-up time of only 1·8 years, but was very large (n=16 485). Our sample size of 1026 participants and short follow-up time might have limited our study power to detect significant differences in FEV1 slope, and there was evidence that our truncated follow-up time probably decreased the precision of the slope measures (see appendix for more discussion). Our data exclude, with 95% confidence, treatment differences in FEV1 slope of more than 39 mL/year in smokers and 29 mL/year in non-smokers, but we were unable to confidently exclude smaller treatment effects. We also could not exclude the possibility that differences might have emerged with longer follow-up.
In conclusion, in treatment-naive, HIV-positive patients with baseline CD4 T-cell counts of more than 500 per µL, immediate initiation of ART seems to have no major effect on short-term rate of lung function decline compared with deferred ART.
Supplementary Material
Research in context.
Evidence before this study
HIV-1 seems to increase the risk for COPD, independent of cigarette smoking status. Although the mechanisms for this increased risk are not clear, use of antiretroviral therapy (ART) has been postulated to potentially reduce COPD risk by decreasing pulmonary inflammatory responses. Conversely, immune reconstitution from ART has also been postulated to increase COPD risk. We searched PubMed for English-language publications containing the terms “HIV” and “chronic obstructive pulmonary disease”, restricted to studies published after 1996 when combination ART became available. Our search revealed contradictory data regarding the effect of ART on risk of COPD. Findings from two studies showed a higher risk of COPD with ART use, one study showed a lower risk of COPD with ART use, and four studies showed no association. All these studies were observational and therefore subject to confounding.
Added value of this study
We enrolled 1026 HIV-positive adults naive to ART with CD4 T-cell counts of more than 500 per µL who were then randomly assigned to a strategy of either immediate ART or deferral of ART until either CD4 T-cell counts decreased to 350 per µL or AIDS developed. Participants were enrolled from both high-income countries and low-to-middle-income countries. We measured lung function before randomisation, and then annually. We showed no difference in lung function decline between the two ART strategies.
Implications of all the available evidence
Our data suggest that in HIV-positive individuals who are naive to ART with CD4 T-cell counts of more than 500 per µL, immediate ART causes neither harm nor benefit to lung function decline. In light of updated WHO recommendations that all HIV-positive individuals should be treated with ART, regardless of their CD4 T-cell count, our results suggest an absence of significant pulmonary harm with such an approach, and that timing of ART initiation has no major short-term effect on lung function decline.
Acknowledgments
The study team expresses our immense gratitude to each of the individual 1026 START Pulmonary Substudy participants for their contributions to our scientific understanding of lung disease in HIV. A full listing of START Pulmonary Substudy team members is included in the appendix and a full listing of the parent START study team members can be found in the primary results publication. The START Pulmonary Substudy reported here was supported by the National Heart Lung and Blood Institute (R01 HL096453); the parent START trial was primarily supported by the National Institute of Allergy and Infectious Diseases Division of AIDS (UM1 AI068641 and UM1 AI120197) with additional support from Agence Nationale de Recherches surle SIDA et les Hipatites Virales (France), the Danish National Research Foundation, the German Ministry of Education and Research, the European AIDS Treatment Network, the Australian National Health and Medical Research Council, and the UK Medical Research Council and National Institute for Health Research. The Veterans Health Administration Office of Research and Development also provided protected research time in support of this study. The University of Minnesota served as sponsor of the study. Permission to use the St George’s Respiratory Questionnaire for COPD was granted to investigators by Paul Jones (St George’s, University of London, London, UK). Antiretroviral drugs were donated by AbbVie, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline/ViiV Healthcare, Janssen Scientific Affairs, and Merck. The views expressed in this article are those of the authors and do not reflect the views of the US Government, the National Institutes of Health, the Department of Veterans Affairs, the funders, or any of the authors’ affiliated academic institutions.
Declaration of interests
KMK, DENie, GC, BA, NBA, EB, AC, EE, SE, EBF, EF, RMI, CMK, JSM, DENix, ET, RW, and JEC received grant support from the National Institutes of Health (R01 HL096453, UM1 AI068641, and UM1 AI120197) for doing this study. Outside this study: DENie has received personal fees from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline; GMC has received personal fees from Gilead Sciences and ViiV Healthcare; AC has received other support from Bristol-Myers Squibb, Gilead Sciences, and Janssen; JSM has received grants from Bristol-Myers Squibb, Pfizer and ViiV Healthcare, and personal fees from Gilead Sciences, MSD, Pfizer, and Stendhal; and JV reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Glaxo Smith Kline, and Novartis. SE has received grant support from the Australian National Health and Medical Research Council and ViiV Healthcare, and personal fees from Merck Research Laboratories and Mylan Laboratories.
Footnotes
See Online for appendix
Contributors
KMK conceived the study. KMK, DENie, JEC, DENix, ET, and JV designed the study. KMK obtained funding. BA, NBA, EB, AC, EE, SE, EBF, EF, RMI, CMK, JSM, DENix, ET, and RW acquired the data. GC and JEC did the primary statistical analyses. KMK drafted the manuscript. KMK, DENie, GC, BA, NBA, EB, AC, GMC, EE, SE, EBF, EF, RMI, CMK, JSM, DENix, ET, JV, RW, and JEC critically revised the manuscript for important intellectual content and approved the final manuscript. KMK, DENie, GC, BA, NBA, EB, AC, GMC, EE, SE, EBF, EF, RMI, CMK, JSM, DENix, ET, JV, RW, and JEC take responsibility for the integrity of the data and the accuracy of the data analysis.
See Online/Comment http://dx.doi.org/10.1016/S2213-2600(16)30329-0
References
- 1.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2:583–592. doi: 10.1016/S2213-2600(14)70017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8:320–325. doi: 10.1513/pats.201006-045WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raynaud C, Roche N, Chouaid C. Interactions between HIV infection and chronic obstructive pulmonary disease: clinical and epidemiological aspects. Respir Res. 2011;12:117. doi: 10.1186/1465-9921-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond MB, Kunisaki KM, Huang L. Obstructive lung diseases in HIV: a clinical review and identification of key future research needs. Semin Respir Crit Care Med. 2016;37:277–288. doi: 10.1055/s-0036-1578801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond MB, Kirk GD, Astemborski J, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67:309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond MB, Merlo CA, Astemborski J, et al. The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. AIDS. 2013;27:1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madeddu G, Fois AG, Calia GM, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection. 2013;41:347–353. doi: 10.1007/s15010-012-0330-x. [DOI] [PubMed] [Google Scholar]
- 11.Pefura-Yone EW, Fodjeu G, Kengne AP, Roche N, Kuaban C. Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir Med. 2015;109:247–254. doi: 10.1016/j.rmed.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(suppl 1):S5–S36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Babiker AG, Emery S, et al. Demographic and HIV-specific characteristics of participants enrolled in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(suppl 1):30–36. doi: 10.1111/hiv.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunisaki K, Niewoehner Collins G, et al. Pulmonary function in an international sample of HIV-positive, treatment-naive adults with CD4 counts >500 cells/µL: a substudy of the INSIGHT Strategic Timing of Anti Retroviral Treatment (START) trial. HIV Med. 2015;;16(suppl S1):119–128. doi: 10.1111/hiv.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundgren JD, Babiker AG, et al. INSIGHT start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standardization of spirometry 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St George Respiratory Questionnaire. Chest. 2007;132:456–463. doi: 10.1378/chest.06-0702. [DOI] [PubMed] [Google Scholar]
- 19.Jones PW. St George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 20.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 21.Danel C, Moh R, et al. TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 23.Samperiz G, Guerrero D, Lopez M, et al. Prevalence of and risk factors for pulmonary abnormalities in HIV-infected patients treated with antiretroviral therapy. HIV Med. 2014;15:321–329. doi: 10.1111/hiv.12117. [DOI] [PubMed] [Google Scholar]
- 24.Leung JM, Liu JC, Mtambo A, et al. The determinants of poor respiratory health status in adults living with human immunodeficiency virus infection. AIDS Patient Care STDS. 2014;28:240–247. doi: 10.1089/apc.2013.0373. [DOI] [PubMed] [Google Scholar]
- 25.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 26.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 27.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.