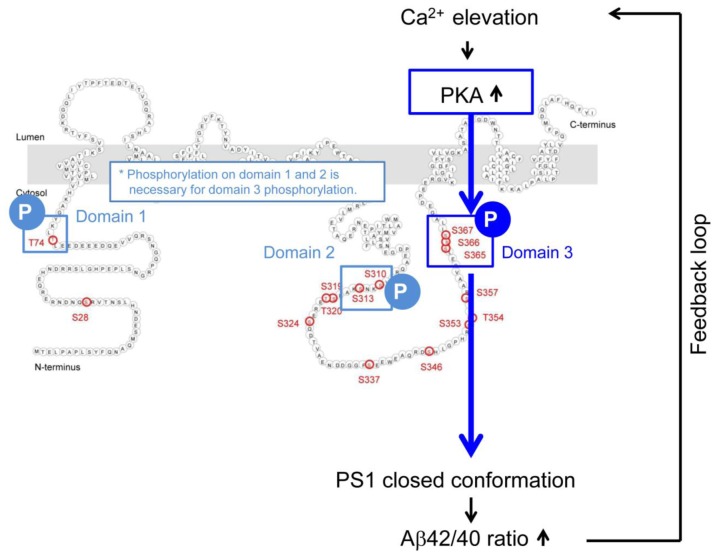
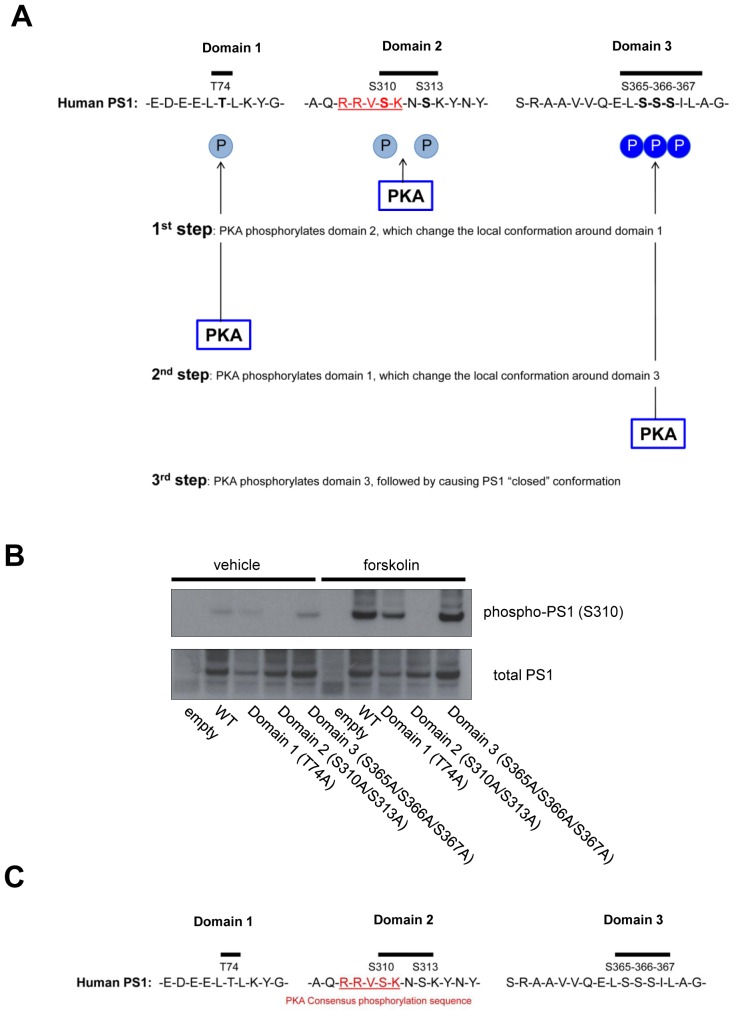
Figure 6. Mechanism of the Ca2+-triggered PS1 pathogenic conformational change.
The schematic image of the molecular events involved in the Ca2+-triggered pathogenic ‘closed’ conformational change and increase of the Aβ42/40 ratio. The elevated Ca2+ levels induce PKA activation, followed by the phosphorylation of PS1 at domain 1, domain 2 and domain 3. Domain 3 phosphorylation, particularly at S367, induces the PS1 pathogenic conformation that leads to increase in the Aβ42/40 ratio.