Abstract
Patients needing hemodialysis are advised to have arteriovenous fistulas rather than catheters because of significantly lower mortality rates. However, disparities in fistula placement raise the possibility that patient factors have a role in this apparent mortality benefit. We derived a cohort of 115,425 patients on incident hemodialysis ≥67 years old from the US Renal Data System with linked Medicare claims to identify the first predialysis vascular access placed. We compared mortality outcomes in patients initiating hemodialysis with a fistula placed first, a catheter after a fistula placed first failed, or a catheter placed first (n=90,517; reference group). Of 21,436 patients with a fistula placed first, 9794 initiated hemodialysis with that fistula, and 8230 initiated dialysis with a catheter after failed fistula placement. The fistula group had the lowest mortality over 58 months (hazard ratio, 0.50; 95% confidence interval, 0.48 to 0.52; P<0.001), with mortality rates at 6, 12, and 24 months after initiation of 9%, 17%, and 31%, respectively, compared with 32%, 46%, and 62%, respectively, in the catheter group. However, the group initiating hemodialysis with a catheter after failed fistula placement also had significantly lower mortality rates than the catheter group had over 58 months (hazard ratio, 0.66; 95% confidence interval, 0.64 to 0.68; P<0.001), with mortality rates of 15%, 25%, and 42% at 6, 12, and 24 months, respectively. Thus, patient factors affecting fistula placement, even when patients are hemodialyzed with a catheter instead, may explain at least two thirds of the mortality benefit observed in patients with a fistula.
Keywords: hemodialysis access, mortality, mortality risk, arteriovenous fistula, dialysis, access
The general recommendation for vascular access in patients on hemodialysis has been fistula first,1–3 although recently, emphasis has been placed on a catheter last strategy.4,5 Much of the encouragement for fistula first, catheter last has been motivated by the large survival advantage reported for patients dialyzed with a fistula rather than a catheter.6–9 Another strong force has been economically motivated by the possible penalties of reduced Medicare reimbursement10 and lower five star ratings11 exacted on dialysis facilities and hospitals for having too few fistulas or too many catheters. Even in the elderly, studies have shown that patients with arteriovenous fistulas (AVFs) have better survival compared with those with central venous catheters (CVCs), with the next best vascular access being arteriovenous grafts.6,7,12 We have reported previously that patients ≥67 years old initiating hemodialysis with a CVC as the first predialysis access placed were associated with a hazard ratio (HR) for mortality of 1.77 (95% confidence interval [95% CI], 1.73 to 1.81) compared with an AVF placed first (P<0.001).7 However, that study offered indications that patient factors, which might create selection bias involving those receiving an AVF rather than a CVC, may have played a significant role in mortality outcomes. For instance, we and others7,13,14 have reported that patients of older age, women, black race, urban location, shorter duration of pre–ESRD nephrology care, or a history of diabetes, malignancy, cerebrovascular disease, or cardiac failure are all associated with lower odds of having an AVF versus a CVC as the initial predialysis vascular access placement. Because no randomized, controlled study has been done or is ever likely to be done to assess what effect these disparities might have on outcome of an AVF versus a CVC, we proposed an alternative observational study that could examine the effect of patient factors, at least in part. In the prior study cited, only 46% of AVFs placed initially were used at hemodialysis initiation, and 43% of the patients started hemodialysis with a CVC.7 Therefore, we questioned whether such patients, although dialyzed with a CVC but who had initially been selected for placement of an AVF, may have better survival rates than those patients who received a CVC as their first hemodialysis access.
Results
Baseline Characteristics
From a cohort of 115,425 patients on incident hemodialysis ≥67 years old, 21,436 (19%) were identified as having an AVF as the first predialysis vascular access. Of those, 9794 (46%) initiated hemodialysis with the first placed fistula (AVF group), 8230 (43%) initiated hemodialysis with a catheter rather than their first placed fistula (AVF-CVC group), and the remaining 3412 who underwent additional access placements and/or started on hemodialysis with a graft (n=1260) were excluded from this study. The CVC group consisted of 90,517 patients (78% of the total) who initiated hemodialysis with a catheter as their first access placed.
The baseline characteristics and differences between the study groups are listed in Table 1. An AVF was statistically more likely to be placed first before a catheter in younger individuals, whites rather than blacks, men rather than women, and normal and overweight individuals rather than those underweight or obese. Patients with diabetes were more likely to have a fistula placement but initiate hemodialysis with a catheter in the AVF-CVC group, whereas those with congestive heart failure or malignancy were more likely to have a CVC placed first. There was a significant association between duration of pre–ESRD nephrology care and type of initial predialysis vascular access (P<0.001). Of those in the AVF group, 52% of the patients and in the AVF-CVC group, 37% were seen by a nephrologist for >1 year before initiating hemodialysis compared with only 17% with a CVC as first hemodialysis access. Only 3% of the AVF group and 9% of the AVF-CVC group compared with 38% of the CVC group had no predialysis nephrology care. Furthermore, those in the CVC group, compared with the AVF-CVC group and AVF group, were more anemic (hemoglobin 10.1±1.6, 10.4±1.5, and 10.7±1.5 g/dl, respectively; P<0.001) and hypoalbuminemic (3.0±0.7, 3.3±0.6, and 3.5±0.6 g/dl, respectively; P<0.001).
Table 1.
Baseline characteristics of the vascular access groups in the study population at hemodialysis initiation
| Characteristicsa | AVF Access Group,b n=9794 | AVF-CVC Access Group,b n=8230 | CVC Access Group,b n=90,517 | P Value |
|---|---|---|---|---|
| Age at onset of ESRD, yr | 76.2 (6.0) | 76.2 (6.03) | 77.1 (6.5) | <0.001 |
| Age categories, yr, % | <0.001 | |||
| 67–79 (n=74,997) | 69 | 70 | 64 | |
| 80–89 (n=37,149) | 29 | 29 | 33 | |
| >90 (n=3279) | 1 | 1 | 3 | |
| Race, % | ||||
| White | 81 | 77 | 76 | <0.001 |
| Black | 15 | 20 | 20 | <0.001 |
| Native American | 1 | 1 | 1 | 0.17 |
| Asian | 4 | 3 | 4 | 0.08 |
| Other | 0.02 | 0.1 | 0.1 | 0.17 |
| Sex, % | <0.001 | |||
| Women | 36 | 45 | 48 | |
| Men | 64 | 55 | 52 | |
| Body mass index, kg/m2 | 27.6 (6.3) | 28.1 (6.6) | 27.1 (6.8) | <0.001 |
| Body mass index categories, kg/m2, % | <0.001 | |||
| Underweight, <18.5 | 4 | 4 | 7 | |
| Normal, 18.5–24.9 | 34 | 31 | 37 | |
| Overweight, 25–29.9 | 35 | 33 | 30 | |
| Obese, ≥30 | 28 | 32 | 27 | |
| Serum creatinine, mg/dl | 5.6 (2.0) | 5.5 (2.1) | 5.5 (2.7) | 0.004 |
| Hemoglobin, g/dl | 10.7 (1.5) | 10.4 (1.5) | 10.1 (1.6) | <0.001 |
| Albumin, g/dl | 3.5 (0.6) | 3.3 (0.6) | 3.0 (0.7) | <0.001 |
| GFR, ml/min per 1.73 m2 | 11.2 (4.1) | 11.2 (4.3) | 11.7 (5.3) | <0.001 |
| Comorbidity index | 7.5 (1.4) | 7.7 (1.4) | 7.8 (1.4) | <0.001 |
| Individual comorbidities, % | ||||
| Diabetes | 52 | 61 | 55 | <0.001 |
| Diabetic end organ disease | 42 | 49 | 40 | <0.001 |
| Cerebrovascular disease | 11 | 12 | 13 | <0.001 |
| Coronary artery disease | 31 | 32 | 30 | <0.001 |
| Congestive heart failure | 32 | 40 | 46 | <0.001 |
| Hypertension | 89 | 88 | 83 | <0.001 |
| Malignancy | 11 | 10 | 12 | <0.001 |
| Peripheral vascular disease | 18 | 19 | 18 | 0.07 |
| COPD | 10 | 11 | 14 | |
| Duration of pre–ESRD nephrology care, mo, % | <0.001 | |||
| No care | 3 | 9 | 38 | |
| 0–6 | 14 | 14 | 10 | |
| 6–12 | 28 | 31 | 20 | |
| >12 | 52 | 37 | 17 | |
| Missing data | 4 | 9 | 15 | |
| Primary cause of ESRD, % | <0.001 | |||
| Diabetes | 42 | 49 | 39 | |
| GN | 7 | 5 | 4 | |
| Hypertension | 38 | 36 | 36 | |
| Cystic disease | 3 | 2 | 1 | |
| Other | 11 | 9 | 20 |
COPD, chronic obstructive pulmonary disease.
Continuous variables are expressed as means±SD, and categorical variables are expressed as percentages of total.
The AVF access group consists of those patients with an AVF placed first but initiating hemodialysis with the AVF. The AVF-CVC access group had an AVF placed first but initiated hemodialysis with a catheter. The CVC access group had a catheter placed first and initiated hemodialysis with the catheter.
Survival Analyses
Entire Study Population Analyses
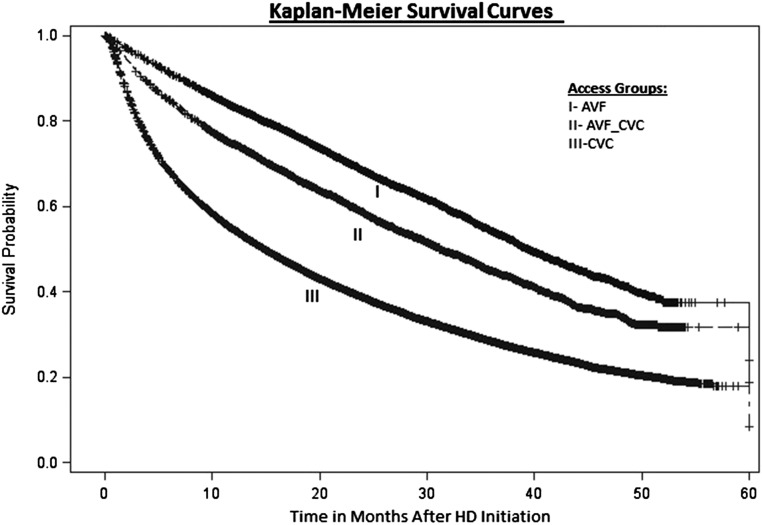
The proportional hazards models were adjusted for potential confounding factors (as listed in Concise Methods), with the CVC group used as the reference in the analyses. As expected, those patients in the AVF group had the lowest mortality rates over the 58-month study period (HR, 0.50; 95% CI, 0.48 to 0.52; P<0.001) compared with those in the CVC group (Figure 1, Table 2). However, those patients with a catheter placed after an AVF as the initial predialysis access (AVF-CVC group) also had a much lower mortality rate over the 58-month study period than those with an initial CVC (HR, 0.66; 95% CI, 0.64 to 0.68; P<0.001). When mortality was examined at times closer to hemodialysis initiation, those patients in the AVF group had 9%, 17%, and 31% deaths at 6, 12, and 24 months, respectively, whereas the AVF-CVC group had 15%, 25%, and 42% deaths, respectively, compared with 32%, 46%, and 62%, respectively, in the CVC group. Thus, analyzing these data suggests that those patients initiating hemodialysis with a catheter after placement of an AVF (the AVF-CVC group) experienced 53% (calculated as [32−15]/32%), 46%, and 32% less mortality at 6, 12, and 24 months, respectively, than those in the CVC group, whereas those starting with an AVF experienced 72%, 63%, and 50% less mortality, respectively. Comparing these mortality rates and HRs suggests that patients receiving AVFs predialysis but starting hemodialysis with CVCs in the AVF-CVC group experienced about 64%–74% of the benefit of a lower mortality risk of those starting with the AVF compared with those in the CVC group.
Figure 1.
Kaplan–Meier survival curves over 58 months after initiating hemodialysis (HD) by vascular access groups. The AVF group (line I) had an AVF placed first and initiated HD with the AVF, the AVF-CVC group (line II) had an AVF placed first but initiated HD with a catheter, and the CVC group (line III) had a catheter placed first and initiated HD with the catheter.
Table 2.
The overall HRs for mortality over the 58-month study period and the 6-, 12-, and 24-month mortality rates in the various vascular access groups
| Event Rates | AVFa Access Group, n=9794 | AVF-CVCa Access Group, n=8230 | CVCa Access Group, n=90,517 |
|---|---|---|---|
| HRb over 58-mo study (95% CI); P value | 0.50 (0.48 to 0.52); P<0.001 | 0.66 (0.64 to 0.68); P<0.001 | 1.0 |
| 6-mo Mortality, no. died/no. at risk (%) | 849/9794 (9%) | 1241/8230 (15%) | 28,641/90,517 (32%) |
| 12-mo Mortality, no. died/no. at risk (%) | 1531/9190 (17%) | 1963/7717 (25%) | 38,387/84,158 (46%) |
| 24-mo Mortality, no. died/no. at risk (%) | 2043/6514 (31%) | 2336/5613 (42%) | 36,563/58,715 (62%) |
Calculated with the CVC group as reference, the Cox proportional hazards models were adjusted for the following covariates: age at hemodialysis initiation, sex, race, comorbidity index, body mass index, cause of ESRD, and duration of pre–ESRD nephrology care.
The AVF access group consists of those patients with an AVF placed first but initiating hemodialysis with the AVF. The AVF-CVC access group had an AVF placed first but initiated hemodialysis with a catheter. The CVC access group had a catheter placed first and initiated hemodialysis with the catheter.
When the HR was calculated using each of the individual comorbidities in the analysis as a separate covariate rather than using the comorbidity index, the results were almost identical: AVF group: HR, 0.51; 95% CI, 0.49 to 0.53; P<0.001 and AVF-CVC group: HR, 0.67; 95% CI, 0.64 to 0.69; P<0.001.
Subgroup Analyses
We recognized that a significant factor affecting the outcome in this analysis may be the lack of data about the vascular access used after initiation of hemodialysis. Thus, the patients in the AVF-CVC group may have transitioned to an AVF sooner than those in the CVC group as one explanation for their better survival. To assess this, we compared the 3126 patients in the AVF-CVC group (38% of the group) whose AVF placement time was >4 months before initiation of hemodialysis with those in the CVC group. Because an AVF that is unusable even at 2 months after creation has usually failed,15 this subgroup with a presumed failed AVF placement >4 months predialysis (mean time of AVF placement of 11.3±6.8 months predialysis) would likely require creation of a new permanent access (or interventional procedure with subsequent maturation of the AVF). Thus, this subgroup should be similar to those in the CVC group, at least in regard to that patients in both groups would require access placement and maturation after initiating hemodialysis. However, the mortality rate of this AVF-CVC subgroup was identically lower than the CVC group over the 58-month study period (HR, 0.65; 95% CI, 0.61 to 0.68; P<0.001), with 6-, 12-, and 24-month mortality rates of 14%, 24%, and 41%, respectively (Supplemental Table 1). Furthermore, analyzing those patients in the AVF-CVC group whose AVF had been placed <4 months or >2, >4, >6, or >9 months predialysis all had similar, beneficially lower mortality rates (HR, 0.64–0.66; P<0.001) compared with the CVC group (Supplemental Table 1).
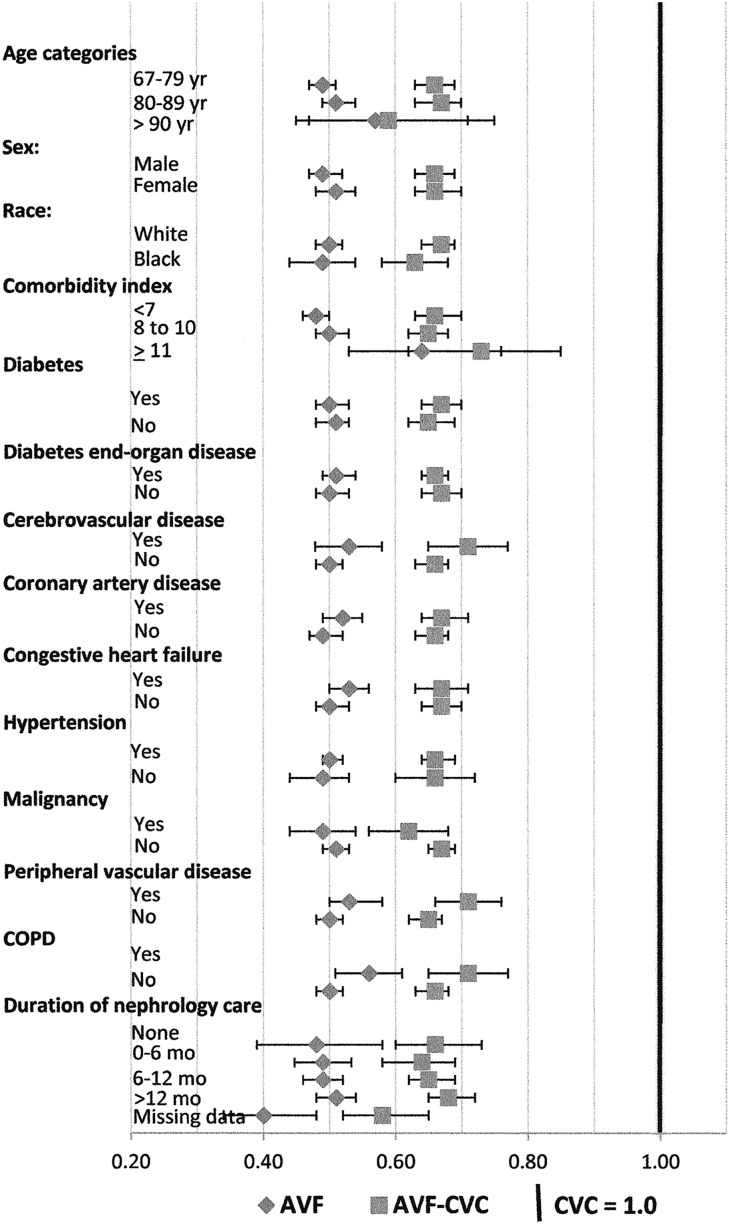
Analysis was further stratified by individual age and comorbidities as shown in Figure 2 (Supplemental Table 2). The AVF-CVC group had markedly lower mortality rates than the CVC group, with HRs of ≤0.65–0.68 (P<0.001) in patients of ages from 67–79 to >90 years old; both men and women; whites and blacks; those with or without diabetes, diabetic end organ disease, coronary artery disease, congestive heart failure, or malignancy; and those with each duration of pre–ESRD nephrology care before hemodialysis initiation. Even in those patients with peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, or a comorbidity index of >10, those in the AVF-CVC group had HRs of ≤0.73 (P<0.001). The AVF group had the lowest HRs in all subgroups, but again, the AVF-CVC group had HRs that suggest that two thirds or more of the mortality benefit experienced by patients in the AVF group may be associated with selection bias on the basis of factors affecting patients receiving an AVF, although they were dialyzed through a catheter.
Figure 2.
Proportional hazard analysis of mortality for vascular access subgroups on the basis of age, sex, race, and comorbidities, with the CVC group as reference. The AVF access group (diamonds) consists of those patients who had an AVF placed first and initiated hemodialysis with the AVF. The AVF-CVC access group (squares) had an AVF placed first but initiated hemodialysis with a catheter. The CVC access group (vertical bold line indicates HR, 1.0) had a catheter placed first and initiated hemodialysis with the catheter. Bars denote 95% CIs. The results shown were derived from 40 separate Cox proportion hazard models, each adjusted for the following covariates: age at ESRD onset, race, sex, body mass index, primary cause of ESRD, duration of pre–ESRD nephrology care, and comorbidity index. In subgroup analyses by comorbidities, the corresponding comorbidity was removed from the comorbidity index calculation to avoid potential collinearity (e.g., in subgroup analysis of diabetes, diabetes was not included in the comorbidity index calculation). Numerical values for the HRs are shown in Supplemental Table 2. COPD, chronic obstructive pulmonary disease.
Discussion
It is well recognized that, among patients receiving maintenance hemodialysis, vascular access poses serious problems. In reported comparisons, patients initiating hemodialysis with a catheter have greater mortality rates of 70%6 and 77%7 more than those initiating with an AVF, promoting a strong emphasis on the fistula first, catheter last initiative. In addition, the recent Medicare Quality Incentive Program imposes penalties of reduced reimbursement10 and lower five star ratings16 on dialysis providers that have too few fistulas or too many catheters. Multiple websites for patient and professional information, such as that of the National Institute of Diabetes and Digestive and Kidney Diseases, promote only an AVF or graft for permanent hemodialysis access.17 In a nephrology forum,18 a vascular catheter was labeled as the “access of evil,” a thought frightening to contemplate as a viable option.18 However, a significant number of patients with ESRD has poor vasculature, leading to inadequate fistula or graft function or arterial steal syndromes, or they are being strongly advised to undergo multiple unsuccessful surgical and/or interventional procedures to achieve a patent access. Furthermore, an additional number of patients has short predicted survival times, fistula arm swelling, or prolonged bleeding periods after each dialysis, which compromise their quality of life. Others may have morbid fear of needles, desire daily short hemodialysis treatments, or soon, use the experimental wearable artificial kidney,19 for which catheters offer obvious advantages. Recent publications20–24 have begun to question fistula first, catheter last for every patient, emphasizing the need for a more balanced, individualized approach to access placement. Therefore, we questioned whether the data demonizing catheters on the basis of their increased mortality may not tell the whole story.
Because disparities in placement of an AVF have been well documented,7,13,14 the possibility exists that patient factors might explain a significant percentage of the increased risk of catheters rather than the catheter itself. It would be helpful to know the real risk of a catheter to counsel patients on hemodialysis with evidence-based options for decisions about surgical and interventional placement or rescue maintenance of an AVF (or graft) versus a catheter. Lacking controlled data or even the likelihood of ever obtaining such data, our observational study compared 8230 patients with a catheter used to initiate hemodialysis after placement of an AVF that had failed or matured too slowly (the AVF-CVC group) with 90,517 patients with a catheter at hemodialysis initiation who were not subject to the selection bias of an AVF placement (the CVC group). The differences between these two groups at baseline were similar to those in prior reports,7,13,14 with those patients receiving an AVF first in the AVF-CVC group including a greater percentage of younger individuals, men, whites, those who were normal or overweight by body mass index rather than underweight or obese, and those who had more predialysis nephrology care, a factor associated with better survival on dialysis25–29 than those in the CVC group. Of note, the serum albumin and hemoglobin levels were significantly higher in the AVF group followed by the AVF-CVC group and then, the CVC group, factors associated with the first and fifth strongest predictors of survival on hemodialysis.8 Therefore, perhaps it should be no surprise that those with an AVF or selected for an initial AVF but starting hemodialysis with a CVC access had better survival than those with an initial CVC. However, we found the degree to which these patient factors seem to affect mortality rates much greater than expected, with the AVF-CVC group having an HR of 0.66 (95% CI, 0.64 to 0.68; P<0.001) over 58 months of hemodialysis follow-up compared with the CVC group. Moreover, the mortality rates of the AVF-CVC group were only 48%, 56%, and 67% of the mortality rate of the CVC group at 6, 12, and 24 months, respectively, after hemodialysis initiation. By comparison of these results with those from patients starting hemodialysis with their fistula in the AVF group (with an HR of 0.50 and mortality rates of 28%, 37%, and 50% of the mortality rate of the CVC group at 6, 12, and 24 months, respectively), it seems that patient factors involved in those receiving an AVF, even if dialyzed with a catheter, are likely to account for about two thirds of the reduced mortality seen in those dialyzed with a fistula compared with a catheter. With recent advances in catheter management that have steadily decreased catheter-associated bacteremia,30–33 these findings make opting for a catheter even more acceptable in certain subgroups of patients, although there is controversy whether catheter-associated bacteremia may be less34 or more35 frequent in elderly patients.
There are certain limitations to our study. First, to obtain Medicare data predialysis, we were limited to patients ≥67 years old. Therefore, these results may be less applicable to younger individuals, but because 50% of incident patients who initiated hemodialysis in the United States in 2013 were >65 years old,36 our data should apply to most of those receiving hemodialysis. Second, we were limited to the available data in US Renal Data System (USRDS)/Medicare claims derived from Centers for Medicare and Medicaid Services (CMS) Form 2728, and therefore, we have no information as to whether or when patients in each of our groups may have converted to an alternative vascular access. It has been reported that conversion from a catheter to a permanent vascular access in patients on incident hemodialysis had an adjusted 30% lower risk of death over 1 year of follow-up,37 and in another study, prevalent patients had an adjusted 31% lower risk over an 8-month period.38 Both studies had significant disparities between converters and nonconverters, consistent with our study. Moreover, when we limited our analysis to those with catheters after unsuccessful fistulas that were placed >4 months before hemodialysis initiation (thus, most AVFs were likely not useable), even these patients had a similar mortality benefit over the patients with catheters (despite the possibility of poor vasculature that might have predisposed to poor outcomes). Survival bias by definition exists in our study, because to be entered in the USRDS, one must survive to the time of initiation of RRT. It has been reported that 17% of those >70 years old39 and 11% of those of all ages40 with a fistula placement died rather than initiating dialysis. The higher chance of dying with predialysis placement of an AVF might favor the AVF and the AVF-CVC groups that have survived to the time of hemodialysis initiation over the CVC group. However, it is notable that those patients with their failed AVFs placed either <4 months or at time periods from >2 to >9 months predialysis experienced the same but no more benefit compared with those in the CVC group, despite the increased time before hemodialysis initiation to allow more deaths to occur, which would have created survival bias. Therefore, it may be more likely that other factors (e.g., urgent crash hemodialysis starts, lack of predialysis nephrology care, or congestive heart failure) play a greater role accounting for the poorer survival of patients with catheters. Third, given the retrospective and observational nature of our study, our conclusion that selection bias of patients for AVF placement, although dialyzed with a catheter, is the most likely explanation of their better survival may be in error. However, the strengths of this study are its large sample size and overall accuracy of the data. Our primary outcome of all-cause mortality is captured accurately from the USRDS data with minimal loss of follow-up along with the use of Medicare claims to help identify our primary variables of interest.
In conclusion, although this study has confirmed the approximately 50% decreased mortality experienced by patients initiating hemodialysis with an AVF compared with a catheter, it showed that about two thirds or more of this mortality benefit is also seen in those initiating hemodialysis with a catheter but who had previously undergone unsuccessful fistula placement. Thus, these observational data suggest that the actual mortality attributed to the catheter itself is much less than previously considered and may be explained in large part by differences in patient factors. Patients with difficulty or complications associated with creation or maintenance of an AVF (or graft) should be given more nuanced data of the actual risks associated with hemodialysis catheters to allow an informed choice of access. For vascular access counseling, we now advocate patient first, fistula second, and catheter last.
Concise Methods
Data Sources
We used the USRDS linked with Medicare claims data to identify patients ≥67 years old initiating hemodialysis from January 1, 2005 to December 31, 2008 with Medicare data from 2003, so that all study patients were Medicare eligible 2 years preceding dialysis initiation.7 Medicare claims searched by Common Procedural Terminology (CPT-4) codes for vascular access were used to identify the first predialysis vascular access placed. The USRDS dataset provided patients’ clinical data to describe baseline characteristics (as derived from CMS Form 2728), the vascular access actually used at hemodialysis initiation, exact dates of death, and dates of transplantation.
Study Population
The final study population was assembled on the basis of identifying the hemodialysis access placed initially as either an AVF (CPT-4 claim for AVF creation 36818, 36819, 36820, 36825, and 36821) or a catheter. Because the CPT-4 codes for tunneled dialysis catheter placement were less reliable, we derived our catheter group using data from both the USRDS and the Medicare CPT-4 claims as previously described.7 Inclusion in the CVC group was on the basis of the assumption that catheters would be placed shortly before the time of hemodialysis initiation, and those catheters would be considered as the first access placed if no evidence of a prior AVF or AVG placement was identified. In the catheter cohort, to identify only the ESRD population (rather than those with AKI who recovered renal function), we excluded those with a catheter removal CPT-4 code <30 days from the date of hemodialysis initiation (0.4% of the group).
Primary Outcome
All-cause mortality (time to death) was the primary outcome of interest. Because the time between vascular access placement and dialysis initiation varies, to avoid lead time bias, we measured time to death from the date of hemodialysis initiation. Because data were accumulated until September 30, 2009, the overall study period was 58 months (of 30 days each), with an average follow-up of 23±13 months for the AVF group, 21±14 months for the AVF-CVC group, and 16±13 months for the CVC group. Patients who received a renal transplant after hemodialysis initiation were censored from the analysis at the time of renal transplantation.
Statistical Analyses
Summary statistics are presented as percentages for categorical data and means (±SD) for continuous variables. Differences in baseline characteristics between the groups were tested using the chi-squared test for categorical variables and one-way ANOVA for continuous variables. We compared the mortality of those with an AVF placed as the first predialysis access who initiated hemodialysis with a catheter (the AVF-CVC group) with that of those initiating with their AVF (the AVF group) and those initiating with a catheter who never had an AVF or a graft placement (the CVC group). Cox proportional hazards models adjusted for the following covariates from CMS Form 2728 (age at hemodialysis initiation, sex, race, comorbidity index [described below], body mass index, cause of ESRD, and duration of pre–ESRD nephrology care25–29) were used to study the associations. To adjust for patient comorbidities, we formed a comorbidity coefficient similar to the Charlson comorbidity index. Each of the comorbid conditions available in the dataset shown in Table 1 contributed points toward the composite index, with additional points given for each decade of age >40 years as described elsewhere.41 We previously used similar approaches to describe comorbidities using abbreviated comorbidity indices by Davies et al.42 and Charlson et al.43 by including information available in the USRDS dataset. These abbreviated indices were validated in the past by strong association with clinical outcomes.44,45 We also performed the mortality analyses using each of the individual comorbidities as a separate covariate rather than using the comorbidity index, with almost identical results as shown in Table 2.
The association between the vascular access groups and mortality was analyzed in the entire study population as well as in subgroups on the basis of age, race, sex, comorbidity index, and the specific comorbidities shown in Figure 2. All analyses were performed with SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
The study was fully funded from departmental funds.
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Vascular Access for Hemodialysis and Value-Based Purchasing for ESRD,” on pages 395–397.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016010019/-/DCSupplemental.
References
- 1.NKF-K/DOQI : III. NKF-K/DOQI clinical practice guidelines for vascular access: Update 2000. Am J Kidney Dis 37[Suppl 1]: S137–S181, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lacson E Jr., Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM: Balancing fistula first with catheters last. Am J Kidney Dis 50: 379–395, 2007 [DOI] [PubMed] [Google Scholar]
- 5.End Stage Renal Disease (ESRD) National Coordinating Center (NCC): Fistula First Catheter Last—FFCL. Available at: http://esrdncc.org/ffcl/. Accessed January 4, 2016
- 6.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 7.DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS: Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297–1304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacson E Jr., Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare & Medicaid Services: ESRD Quality Incentive Program. Available at: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/esrdqip/index.html. Accessed January 4, 2016
- 11.Medicare: The Official US Government Site for Medicare. Dialysis Facility Compare. Star Ratings. Available at: https://www.medicare.gov/dialysisfacilitycompare/?mkt_tok=3RkMMJWWfF9wsRovva3PZKXonjHpfsX84%2B4sUbHr08Yy0EZ5VunJEUWy2YMJTcZ0aPyQAgob-Gp5I5FEPSLfYXqlht6wMWg%3D%3D#data/star-ratings-system. Accessed January 4, 2016
- 12.DeSilva RN, Sandhu GS, Garg J, Goldfarb-Rumyantzev AS: Association between initial type of hemodialysis access used in the elderly and mortality. Hemodial Int 16: 233–241, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Patibandla BK, Narra A, Desilva R, Chawla V, Vin Y, Brown RS, Goldfarb-Rumyantzev AS: Disparities in arteriovenous fistula placement in older hemodialysis patients. Hemodial Int 18: 118–126, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Reddan D, Klassen P, Frankenfield DL, Szczech L, Schwab S, Coladonato J, Rocco M, Lowrie EG, Owen WF Jr.; National ESRD CPM Work Group : National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol 13: 2117–2124, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Asif A, Roy-Chaudhury P, Beathard GA: Early arteriovenous fistula failure: A logical proposal for when and how to intervene. Clin J Am Soc Nephrol 1: 332–339, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Medicare: The Official US Government Site for Medicare. Dialysis Facility Compare. Why Quality Measures Are Important to You: Best Treatment Practices. Available at: https://www.medicare.gov/Dialysisfacilitycompare/#about/dialysisfacility-info/quality-measures/best-treatment-practices. Accessed January 6, 2016
- 17.NIH, The National Institute of Diabetes and Digestive and Kidney Diseases: Health Information. Vascular Access for Hemodialysis. Available at: http://www.niddk.nih.gov/health-information/health-topics/kidney-disease/vascular-access-for-hemodialysis/Pages/index.aspx. Accessed December 18, 2015
- 18.Jaber BL: Bacterial infections in hemodialysis patients: Pathogenesis and prevention. Kidney Int 67: 2508–2519, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gura V, Rivara MB, Bieber S, Munshi R, Smith NC, Linke L, Kundzins J, Beizai M, Ezon C, Kessler L, Himmelfarb J: A wearable artificial kidney for patients with end-stage renal disease. JCI Insight 1: e86397, 2016 [DOI] [PMC free article] [PubMed]
- 20.Kliger AS: Quality measures for dialysis: Time for a balanced scorecard. Clin J Am Soc Nephrol 11: 363–368, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drew DA, Lok CE, Cohen JT, Wagner M, Tangri N, Weiner DE: Vascular access choice in incident hemodialysis patients: A decision analysis. J Am Soc Nephrol 26: 183–191, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wish JB: Catheter last, fistula not-so-first. J Am Soc Nephrol 26: 5–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubbs V, Moss AH, Cohen LM, Fischer MJ, Germain MJ, Jassal SV, Perl J, Weiner DE, Mehrotra R; Dialysis Advisory Group of the American Society of Nephrology : A palliative approach to dialysis care: A patient-centered transition to the end of life. Clin J Am Soc Nephrol 9: 2203–2209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo K, Goldman DP, Romley JA: Early failure of dialysis access among the elderly in the era of Fistula First. Clin J Am Soc Nephrol 10: 1791–1798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Stehman-Breen CO, Sherrard DJ, Gillen D, Caps M: Determinants of type and timing of initial permanent hemodialysis vascular access. Kidney Int 57: 639–645, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Astor BC, Eustace JA, Powe NR, Klag MJ, Sadler JH, Fink NE, Coresh J: Timing of nephrologist referral and arteriovenous access use: The CHOICE Study. Am J Kidney Dis 38: 494–501, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, Owen W Jr.: Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 55: 711–716, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa T, Bragg-Gresham JL, Yamazaki S, Fukuhara S, Akizawa T, Kleophas W, Greenwood R, Pisoni RL: Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol 4: 595–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James MT, Conley J, Tonelli M, Manns BJ, MacRae J, Hemmelgarn BR; Alberta Kidney Disease Network : Meta-analysis: Antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med 148: 596–605, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Campos RP, do Nascimento MM, Chula DC, Riella MC: Minocycline-EDTA lock solution prevents catheter-related bacteremia in hemodialysis. J Am Soc Nephrol 22: 1939–1945, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore CL, Besarab A, Ajluni M, Soi V, Peterson EL, Johnson LE, Zervos MJ, Adams E, Yee J: Comparative effectiveness of two catheter locking solutions to reduce catheter-related bloodstream infection in hemodialysis patients. Clin J Am Soc Nephrol 9: 1232–1239, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghaddas A, Abbasi MR, Gharekhani A, Dashti-Khavidaki S, Razeghi E, Jafari A, Khalili H: Prevention of hemodialysis catheter-related blood stream infections using a cotrimoxazole-lock technique. Future Microbiol 10: 169–178, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Murea M, James KM, Russell GB, Byrum GV 3rd, Yates JE, Tuttle NS, Bleyer AJ, Burkart JM, Freedman BI: Risk of catheter-related bloodstream infection in elderly patients on hemodialysis. Clin J Am Soc Nephrol 9: 764–770, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: Incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 36.United States Renal Data System: Volume 2. Chapter 1: Incidence, Prevalence, Patient Characteristics, and Treatment Modalities. Table 1.7 Number and Percentage of Incident Cases of Hemodialysis (HD), Peritoneal Dialysis (PD), and Transplantation by Age, Sex, Race, Ethnicity, and Primary Cause of ESRD, in the U.S. Population, 2013. Available at: http://www.usrds.org/2015/view/v2_01.aspx. Accessed March 14, 2016
- 37.Bradbury BD, Chen F, Furniss A, Pisoni RL, Keen M, Mapes D, Krishnan M: Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis 53: 804–814, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Lacson E Jr., Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Lee T, Thamer M, Zhang Y, Zhang Q, Allon M: Outcomes of elderly patients after predialysis vascular access creation. J Am Soc Nephrol 26: 3133–3140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM: Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol 7: 466–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gueye AS, Baird BC, Shihab F, Koford JK, Barenbaum AL, Leviatov A, Goldfarb-Rumyantzev AS: The role of the economic environment in kidney transplant outcomes. Clin Transplant 23: 643–652, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI: Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: Their interrelationship and prediction of survival. Am J Kidney Dis 26: 353–361, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, Wang Z, Baird B, Barenbaum L, Cheung AK: Duration of end-stage renal disease and kidney transplant outcome. Nephrol Dial Transplant 20: 167–175, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Tang H, Chelamcharla M, Baird BC, Shihab FS, Koford JK, Goldfarb-Rumyantzev AS: Factors affecting kidney-transplant outcome in recipients with lupus nephritis. Clin Transplant 22: 263–272, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.