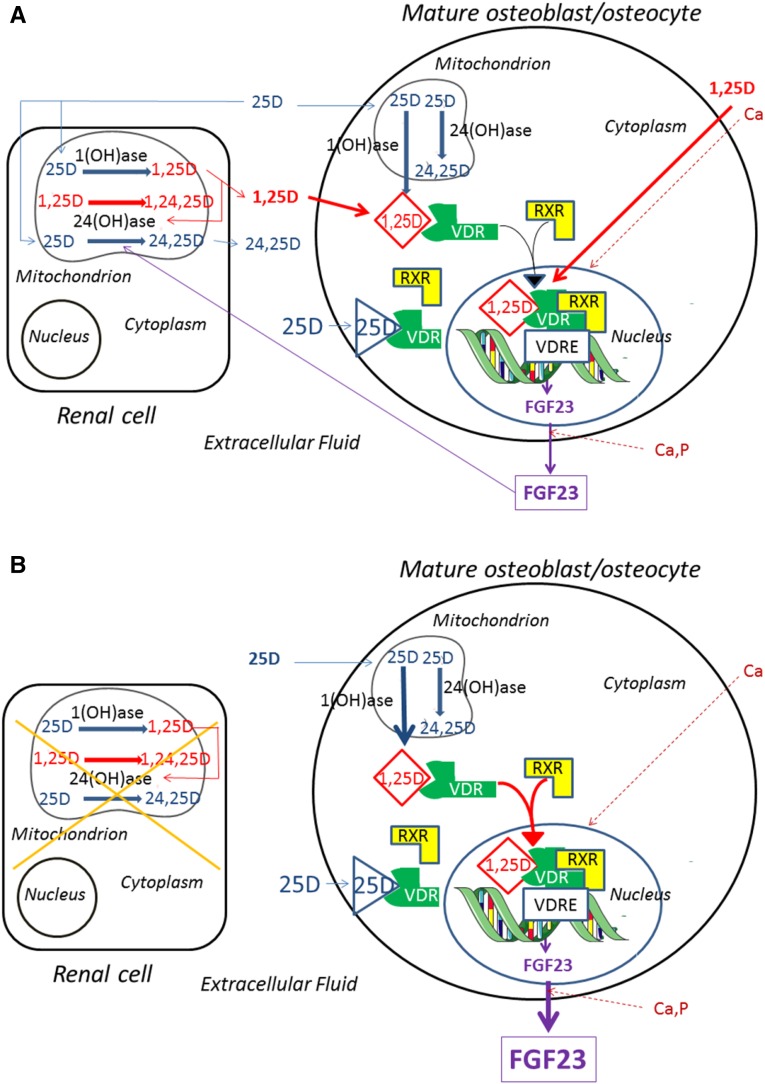
Figure 7.
Model of the regulation of FGF23 by vitamin D metabolites and minerals showing the important role of renal-derived and osteoblast- derived 1,25(OH)2D. (A) In the presence of normal renal function. Circulating 25(OH)D can be converted in the kidney to 1,25(OH)2D via the 1(OH)ase (Cyp27b1). 1,25(OH)2D can stimulate 24(OH)ase and 25(OH)D can be degraded by the 24(OH)ase (Cyp24a1) to 24,25(OH)2D (24,25D). 1,25(OH)2D can also be converted to 1,24,25(OH)3D (1,24,25D) by the 24(OH)ase. 1,25(OH)2D can itself stimulate 24(OH)ase activity. Renal-derived circulating 1,25(OH)2D can then enter the mature osteoblast/osteocyte and bind the VDR which then complexes with the RXR. In the nucleus, this complex may bind to an FGF23 VDRE and increase FGF23 transcription, resulting in increased FGF23 production and release. Circulating 25(OH)D may also enter the mature osteoblast/osteocyte and be converted by the osteoblastic 1(OH)ase to 1,25(OH)2D or be degraded by the osteoblastic 24(OH)ase to 24,25(OH)2D. The intracellular 1,25(OH)2D produced from 25(OH)D by the osteoblast may act as an intracrine factor and bind to VDR and increase FGF23 production. At high circulating concentrations of 25(OH)D, this vitamin D metabolite may also act by directly binding to VDR and stimulating FGF23 production. Both Ca, by unknown mechanisms, and 1,25(OH)2D, by stimulation of transcription, can increase VDR concentrations and facilitate the actions of 1,25(OH)2D and 25(OH)D. Ca and P can increase FGF23 production by unknown mechanisms. (B) In the absence of renal function. Renal conversion of 25(OH)D to 1,25(OH)2D is impaired, and renal degradation of 25(OH)D is also impaired. Osteoblastic production of 1,25(OH)2D becomes the major source of 1,25(OH)2D in the mature osteoblast/osteocyte and a major regulator of FGF23 production. Circulating 25(OH)D levels may be influenced by nutritional intake of vitamin D as well as ultraviolet light–induced production of vitamin D.