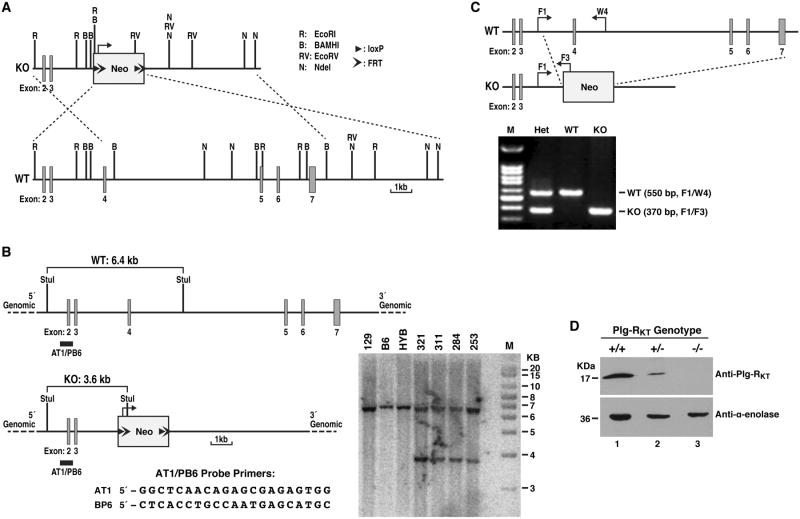
Figure 1. Generation and characterization of Plg-RKT deficient mice.
(A) Construction of a conventional targeting vector for Plg-RKT. An 18.90 kb region was first subcloned from a positively identified 129 BAC clone (RPCI-22: 24B9) using a homologous recombination-based technique. The targeting vector was designed such that the long homology arm extended 5.40 kb 3′ to the 3′ end of a pGK-gb2 loxP/FRT-flanked neomycin cassette. The short homology arm was located on the 5′ side of the neomycin cassette and was 3.08 kb long. An Nde I site was engineered into the neomycin cassette for Southern blot analysis. The targeting vector was confirmed by restriction analysis after each modification step and by sequencing using primers designed to read from the selection cassette into the 3′ end of the short homology arm and the 5′ end of the long homology arm, or from primers that anneal to the vector sequence, and read into the 5′ and 3′ ends of the BAC sub clone. The BAC was subcloned into a 2.4 kb (pSP72, Promega) backbone vector containing an ampicillin selection cassette for retransformation of the construct prior to electroporation. The total size of the targeting construct was 10.18 kb. The pGK-gb2 loxP/FRT-flanked neomycin cassette was inserted into the gene. Exons 4-7 were conventionally targeted and the neomycin cassette replaced 10.42 kb of this gene. The targeting construct was linearized using Not I prior to electroporation into ES cells. Restriction enzyme sites, LoxP and FRT sites are indicated. (B) Southern blotting strategy used to identify ES cells carrying the neomycin cassette. DNA was digested with StuI and hybridized with a probe targeted against the 5′ internal region. The expected sizes are indicated on the schematic. Clones 284, 311, 321 and 253 were confirmed as correctly targeted. DNA from C57Bl/6 (B6), 129/SvEv (129), and C57Bl/6 × 129/SvEv hybrid (HYB) mouse strains were used as wild type controls. Positive clones were injected into C57Bl/6 blastocysts and implanted into pseudopregnant C57Bl/6 females. (C) Polymerase chain reaction-based strategy for identification of Plg-RKT-/- (KO), Plg-RKT+/- (Het) and Plg-RKT+/+ (WT) mice. Mice were genotyped to confirm germline transmission using a polymerase chain reaction- based strategy. A common forward primer (F1, AAGAGGATGAGACCTGTGTCAGAG) in Plg-RKT in the intron between exons 3 and 4 and a reverse primer (W4, GAAGCCGAGCATGCGTAACCATGAAC) in Plg-RKT exon 4 yielded an amplicon of 550 bp for the wild type gene. The forward primer (F1) and the reverse primer (F3, GCATAAGCTTGGATCCGTTCTTCGGA) in the Neo resistance gene provided a 370 bp amplicon only for the targeted gene. Unless otherwise indicated, mice backcrossed 10 generations into C57Bl/6J were used in these studies and maintained in a Helicobacter free room. (D) Western blot analysis was carried out as described [9] on lysates of thioglycollate elicited macrophages from Plg-RKT-/-, Plg-RKT+/- and Plg-RKT+/+ mice using anti-Plg-RKT MAb [8,9]. Anti-α-enolase MAb [30] was used as loading control.