Abstract
This study was conducted to retrospectively investigate the efficacy and safety of pegaspargase, gemicitabine, oxaliplatin and dexamethasone (Peg-GemOD) combination chemotherapy as a first-line therapy for advanced-stage extranodal NK/T cell lymphoma (ENKTL). Eighteen patients with newly diagnosed stage III/IV ENKTL were subjected to 3–6 cycles of Peg-GemOD chemotherapy. After 3 cycles of therapy, the overall response rate was 67 % (12/18) with a complete response rate of 28 % (5/18) and a partial response rate of 39 % (7/18). The median overall survival (OS) and progression-free survival (PFS) time were 10 and 8.5 months respectively. For those responders, the median OS and PFS time were significantly better than those of non-responders (median OS, 15 vs. 10 months; P = 0.001 and median PFS, 15 vs. 7 months; P = 0.001). Furthermore, patients with low plasma EBV-DNA levels after induction chemotherapy had a remarkably longer OS and PFS time. The toxicity of Peg-GemOD regimen was acceptable.
Keywords: Extranodal NK/T cell lymphoma, Induction chemotherapy, Pegaspargase, Gemicitabine, Oxaliplatin, Prognostic factors
Introduction
Extranodal NK/T cell lymphoma (ENKTL) is a distinct subtype of non-Hodgkin’s lymphoma which has a predilection for Asian and South American populations [1]. In China, ENKTL makes up 5–10 % of all malignant lymphomas. Although patients with stage I/II ENKTL can achieve favorable long-term outcome from concurrent or sequential chemoradiotherapy [2, 3], advanced-stage (stage III/IV) ENKTL is still highly invasive, respond unsatisfactorily to conventional combination chemotherapy with extremely low 1-year overall survival (OS) rate. Great efforts have been made to explore optimal systemic chemotherapy for advanced-stage ENKTL. However, there is currently no consensus on standard therapeutic regimens for this formidable disease.
Chemotherapy protocols generally used to treat lymphomas of other histological subtypes have very little efficacy in ENKTL patients with disseminated disease. The mechanisms of ENKTL resistance to conventional chemotherapy might be related to the overexpression of P-glycoprotein in tumor cells, which is responsible for the multi-drug resistance (MDR) phenotype [4]. Recently, several non-MDR-dependent drugs were incorporated in protocols specifically designed for ENKTL. Among these novel protocols, l-asparaginase or its pegylated form pegaspargase-based combination chemotherapy regimens have been observed with promising results in patients with advanced-stage ENKTL [5–7]. In addition, as a nucleoside analogue, gemcitabine alone or its combination regimens have shown considerable efficacy in the treatment of ENKTL, particularly in relapsed or refractory ENKTL patients following l-asparaginase-containing chemotherapy [8]. Based on these observations, chemotherapeutic regimens incorporating both gemcitabine and l-asparaginase or pegaspargase have emerged with favorable outcomes in stage I/II or relapsed/refractory ENKTL patients. However, there have been few studies to examine the treatment outcomes of chemotherapy regimens comprising gemcitabine and pegaspargase for advanced-stage ENKTL. From 2011, we began to use pegaspargase combined with GemOD (gemicitabine, oxaliplatin and dexamethasone) regimen, which has been validated as an effective salvage regimen for patients with relapsed or refractory T cell lymphoma in our previous study [9], to treat patients with newly diagnosed stage III/IV ENKTL as a first-line therapy. In this retrospective study, we firstly evaluated the efficacy and safety profiles of this novel regimen containing pegaspargase, gemicitabine, oxaliplatin and dexamethasone (Peg-GemOD) and prognostic factors in advanced-stage ENKTL.
Methods
Patients
From January 2011 to December 2014, a total of 18 patients with newly diagnosed stage III/IV ENKTL, who received Peg-GemOD regimen as induction chemotherapy for at least 3 cycles, were retrospectively identified. The inclusion criteria of this retrospective study were as follows: (1) pathologically confirmed diagnosis of ENKTL in accordance with the WHO criteria; (2) Immunohistochemistry analysis of neoplastic cells typically positive for cytoplasmic CD3ε, CD56, granzyme B, and TIA-1 but negative for surface CD3, CD79a and CD20; Epstein-Barr virus (EBV)-encoded small nuclear RNA (EBER) detected by in situ hybridization; (3) previously untreated, Ann Arbor stage III/IV ENKTL; (4) received Peg-GemOD regimen as induction chemotherapy for at least 3 cycles; (5) a complete set of clinical information and follow-up data.
Clinical demographics, staging work-up data and prognostic factors were collected including age, sex, systemic B symptoms, Eastern Cooperative Oncology Group performance status (ECOG PS), a complete medical history, physical examination, complete blood count, serum biochemistry with lactate dehydrogenase (LDH), computed tomography (CT) scans or magnetic resonance imaging (MRI) of head, neck, thorax, chest and abdomen, whole-body positron emission tomography-computed tomography (PET-CT), bone marrow examination and plasma EBV-DNA. Bone marrow involvement was defined as lymphoma cells found in specimen or unifocal, multifocal and diffuse fluorodeoxyglucose (FDG) uptake within the skeleton higher than that in the liver on PET-CT images. Plasma EBV-DNA measurement was determined by quantitative polymerase chain reaction (PCR) assay,which was expressed as copies per ml of sample with a minimum detection level of 5 × 103 copies/ml.
Clinical data were retrieved through the hospital electronic medical record system after approval obtained from the Hospital Ethics Committee and conducted in accordance with the precepts established by the Declaration of Helsinki.
Treatment Protocol
The Peg-GemOD regimen consisted of pegaspargase at a dose of 2500 U/m2 intramuscularly on day 1, gemcitabine at a dose of 1000 mg/m2 as a 30 min intravenous infusion on days 1 and 8, oxaliplatin at a dose of 100 mg/m2 diluted in 5 % dextrose solution as a 3 h infusion delivered on day 1 after gemcitabine, and dexamethasone at a dose of 20 mg intravenously on days 1–4. The regimen was repeated every 3 weeks. Pegaspargase was used with no skin test or pre-medication. An anti-emetic protocol including ondansetron or dolasetron was administered before chemotherapy. For patients who experienced grade 3 or more hematologic and/or non-hematologic toxicities except alopecia, the total dose of gemcitabine and oxaliplatin to be administered for all following cycles was reduced by 25 %. Granulocyte colony stimulating factor (G-CSF, 5 μg/kg/day) or recombinant humanized thrombopietin (rhTPO 300 IU/kg/day) were given to patients who developed grade 3–4 neutropenia or thrombocytopenia as supportive therapy. All patients received at least 3 cycles of Peg-GemOD chemotherapy with a maximum of 6 cycles. Salvage chemotherapy or hematopoietic stem cell transplantation was offered to patients who suffered from progressive disease following chemotherapy.
Response and Toxicity Criteria
Response was classified as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to Revised Cheson’s standard response criteria. The treatment response evaluation data included assessment after every 3 cycles of chemotherapy and the completion of treatment. OS was calculated from the date of diagnosis to death from any cause or the last follow-up. Progression-free survival (PFS) was measured from the initiation of chemotherapy to the date of disease progression, relapse, death or last follow-up visit.
All adverse effects after chemotherapy were graded based on version 3.0 of National Cancer Institute Common Terminology Criteria of Adverse Events. Clinical records for evaluation of chemotherapeutic toxicities included the results of laboratory measurements for complete blood cell count, coagulation tests, amylase, glucose, bilirubin, aspartate aminotransferase, and alanine aminotransferase.
Statistical Methods
Survival analysis was performed using the Kaplan–Meier method. The log-rank test was used to compare survival curves. P values less than 0.05 were considered significant. All statistical analyses were performed using SPSS 17.0 software.
Results
Patient Characteristics
Clinical demographics and characteristics of all patients were summarized in Table 1. The median age was 57 years (range 30–70 years). Six patients were older than 60 years of age. There was a slight male predominance, with a male-to-female ratio of 1.25:1. Eleven patients had Ann Arbor stage III ENKTL, and seven patients had stage IV disease. Six patients presented with non-upper aerodigestive tract (NUAT) involvement with dissemination to lymph nodes (n = 4), bone marrow (n = 2), lung (n = 2), testis (n = 1), intestinal tract (n = 1), and skin (n = 4). The ECOG PS scores of six patients were ≥2. Systemic B symptom and elevated lactate dehydrogenase (LDH) were observed in 12 and 13 patients, respectively. Additionally, five patients suffered from hemophagocytic syndrome characterized by fever, hepatosplenomegaly, cytopenias, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia and hemophagocytosis in bone marrow or lymph node. According to the international prognostic index (IPI), all of patients had high-intermediate or high IPI risk scores.
Table 1.
Patient Characteristics (n = 18)
| Characteristics | Patient no. | Percentage |
|---|---|---|
| Gender | ||
| Male | 10 | 56 |
| Female | 8 | 44 |
| Age (years) | ||
| >60 | 6 | 33 |
| ≤60 | 12 | 67 |
| ECOG PS | ||
| 0 | 5 | 28 |
| 1 | 7 | 39 |
| 2 | 6 | 33 |
| B symptoms | ||
| Yes | 12 | 67 |
| No | 6 | 33 |
| Ann Arbor stage | ||
| III | 11 | 61 |
| IV | 7 | 39 |
| IPI scores | ||
| 2 | 3 | 17 |
| 3 | 6 | 33 |
| 4 | 7 | 39 |
| 5 | 2 | 11 |
| LDH level | ||
| Elevated | 12 | 67 |
| Normal | 6 | 33 |
| Hemophagocytic syndrome | ||
| Present | 5 | 28 |
| Absent | 13 | 72 |
| Involved sites | ||
| UAT | 12 | 67 |
| NAT | 6 | 33 |
| Bone marrow | 2 | 33 |
| Skin | 4 | 67 |
| Distant lymph nodes | 4 | 67 |
| Intestinal tract | 4 | 67 |
| Testis | 1 | 17 |
Values are presented as number (%)
UAT upper aerodigestive tract, NUAT non-upper aerodigestive tract
Therapy Delivered and Response Rates
A total of 88 cycles of Peg-GemOD regimen were delivered. The median number of cycles administered was 5 (range 3–6 cycles), with seven patients completing 6 cycles. There were a total of 42 cycle delays. Among them, 32 delays were due to myelosuppression, 10 cycle delays due to elevated liver enzymes. Meanwhile, dose reduction was required in seven patients because of grade 3–4 hematological toxicities, while there was no dose reduction for non-hematological toxicity. Altogether, 46 cycles proceeded without treatment delays and 57 cycles were administered without dose reduction.
The overall results of treatment response were summarized in Table 2. After 3 cycles, the overall response rate (ORR, CR + PR) was 67 % (12 of 18 patients). CR and PR were achieved in 5 of 18 patients and 7 of 18 patients, respectively. In addition, SD was observed in two patients during therapy and PD in four patients. A total of 14 patients with no evidence of progressive disease after 3 cycles of the Peg-GemOD regimen received additional cycles of chemotherapy. Among them, seven patients accomplished 3 additional cycles of the Peg-GemOD regimen while the other 5 died of progressive lymphoma during the following cycles of treatment and one patient died of disease related co-morbidities after 5 cycles of chemotherapy. One patient gave up therapy for personal reason. Five of seven patients who received 6 cycles of the Peg-GemOD regimen responded, including 4 CRs and 1 PR. Two of 7 patients suffered PD. Notably, these two patients lost response despite having achieved CR after 3 cycles of treatment. Analyzing by Ann Arbor stage, we observed that responses were seen in 8 of 11 stage III and 4 of 7 stage IV patients. Of note, patients with primary involved site other than upper aerodigestive tract were poor responders, where two patients died from disease progression and 1 died from sepsis 2 months after achieving a PR. Interestingly, 1 stage III NUAT patient who achieved a PR after 3 cycles continued to improve and achieved a CR after completing six cycles of treatment.
Table 2.
Response to treatment
| Response | Overall no. of patients | Ann Arbor stage | Involved site | ||
|---|---|---|---|---|---|
| III (n) | IV (n) | UAT (n) | NUAT (n) | ||
| Total | 18 | 11 | 7 | 12 | 6 |
| After 3 courses | |||||
| CR | 5 (28 %) | 3 (27 %) | 2 (29 %) | 4 (33 %) | 1 (17 %) |
| PR | 7 (39 %) | 5 (46 %) | 2 (29 %) | 4 (33 %) | 3 (50 %) |
| ORR | 12 (67 %) | 8 (73 %) | 4 (58 %) | 8 (66 %) | 4 (67 %) |
| After 6 courses | |||||
| CR | 4 (22 %) | 3 (27 %) | 1 (14 %) | 3 (25 %) | 1 (17 %) |
| PR | 1 (6 %) | 1 (9 %) | 1 (8 %) | ||
| ORR | 5 (28 %) | 4 (36 %) | 1 (14 %) | 4 (33 %) | 1 (17 %) |
Values are presented as number (%)
CR complete response, PR partial response, ORR overall response rate, UAT upper aerodigestive tract, NUAT non-upper aerodigestive tract
Survival Analysis
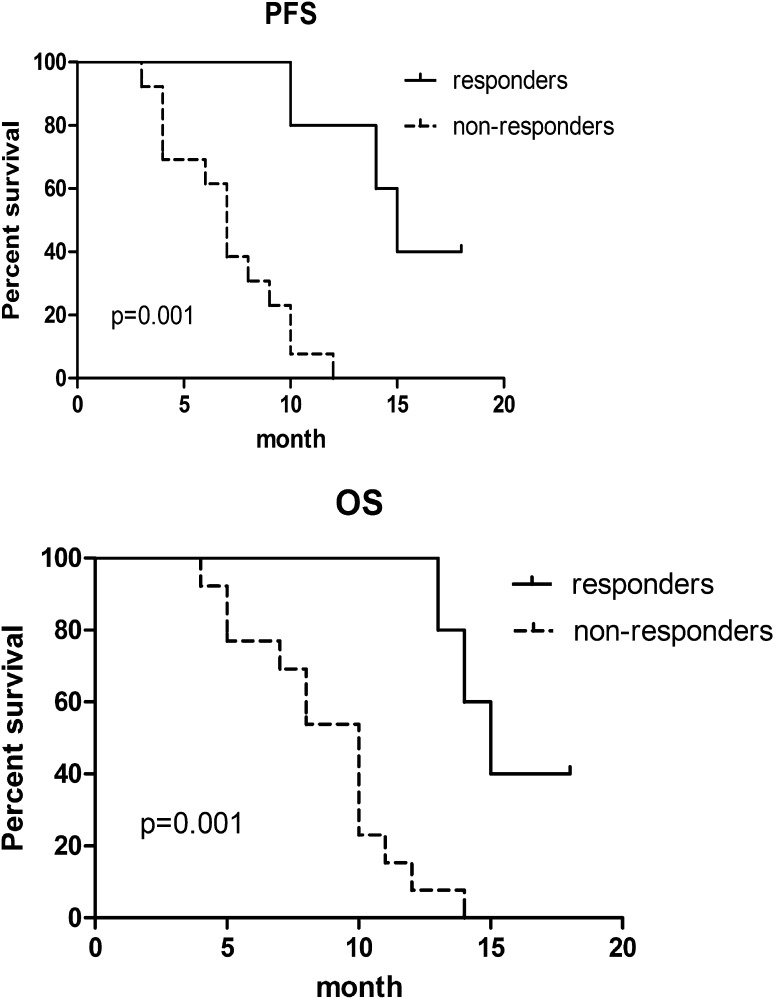
With the median follow-up time of 18 months (range 3–24 months), the median OS and PFS of these patients were 10 and 8.5 months respectively. The OS and PFS of the responders (CR + PR) who received 6 cycles of the Peg-GemOD regimen were significantly better than those of non-responders (median OS, 15 vs. 10 months; P = 0.001 and median PFS, 15 vs. 7 months; P = 0.001) (Fig. 1).
Fig. 1.
Progress-free survival and overall survival of responders and non-responders after treatment with Peg-GemOD regimen
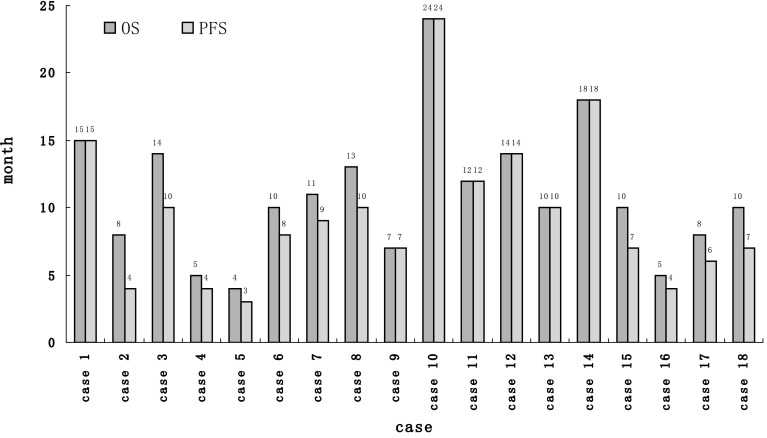
The median EBV-DNA level of 18 patients was 6.62 × 105 copies/ml (range 3.42 × 104–7.26 × 105 copies/ml) before therapy. After 6 cycles of treatment, the plasma EBV-DNA was undetectable (<5×103 copies/ml) in 3 of the 4 patients who achieved CR, and remission was maintained at the time of last follow-up. The median PFS of these patients reached 18 months (Fig. 2). The fourth CR patient had EBV titre of 1.14 × 104 copies/ml at end of treatment and experienced disease recurrence 2 months later.
Fig. 2.
Progress-free survival and overall survival of whole cohort (case 1, case 10 and case 14 with undetectable plasma EBV-DNA following chemotherapy)
At the time of the present analysis, six patients are still alive with 2 in PD, 3 in CR and 1 in PR. Two PD patients received salvage chemotherapy with 1 achieving PR. Autologous hematopoietic stem cell transplantation was performed for 1 CR patient.
Treatment-Related Toxicity
Treatment-related toxicities were acceptable, as detailed in Table 3. The main adverse event was hematological toxicity. Grade 3/4 neutropenia was observed in 63 % of all delivered cycles. Febrile neutropenia complicated 23 % of cycles, where one patient died of neutropenic sepsis. Grade 3/4 thrombocytopenia was recorded in 70 % of cycles, with six patients receiving a median of 2 units (range 1–4) of platelets transfusion. Grade 3/4 anemia occured in 12 % of patients. With regards to non-hematologic toxicity, liver dysfunction presenting as elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was observed in 20 % of cycles, mainly occurring in the first week of chemotherapy. Only one patient developed pancreatitis, which resolved with somatostatin and supportive care. Prolonged activated partial thromboplastin time (APTT) and/or prothrombin time (PT) as well as hypofibrinogenemia were recorded in 32 and 29 % of cycles respectively, but without bleeding or thromboembolism. Other non-hematologic toxicities including gastrointestinal toxicity and hyperglycemia were mild or moderate, which were controlled with supportive care. There was no cases of hypersensitivity or thrombotic events.
Table 3.
Treatment-related toxicities
| Adverse event | Grade of toxicity | |||
|---|---|---|---|---|
| Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) | |
| Hematologic | ||||
| Anemia | 26 (30 %) | 20 (23 %) | 10 (11 %) | 1 (1 %) |
| Leucopenia | 2 (2 %) | 24 (27 %) | 50 (57 %) | 5 (6 %) |
| Thrombocytopenia | 0 | 25 (28 %) | 55 (63 %) | 6 (7 %) |
| Nonhematologic | ||||
| Nausea | 23 (26 %) | 4 (5 %) | 0 | 0 |
| APTT/PT prolongation | 21 (24 %) | 5 (6 %) | 2 (2 %) | |
| Hypofibrinogenemia | 18 (20 %) | 5 (6 %) | 2 (2 %) | 1 (1 %) |
| ALT elevation | 15 (17 %) | 3 (3 %) | 0 | 0 |
| Hyperbilirubinaemia | 6 (7 %) | 2 (2 %) | 0 | 0 |
| Hyperglycemia | 6 (7 %) | 3 (3 %) | 0 | 0 |
| Raised creatinine | 9 (10 %) | 0 | 0 | 0 |
| Paresthesias | 11 (13 %) | 0 | 0 | 0 |
| Palpitations | 7 (8 %) | 0 | 0 | 0 |
Table shows number of cycles affected and percentage of total cycles in which the respective toxicity occurred
APTT activated partial thromboplastin time, PT prothrombin time, ALT alanine aminotransferase
Discussion
Currently, combination chemotherapy remains the mainstay of treatment for advanced-stage ENKTL. l-asparaginase-containing regimens, including SMILE (dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide) and AspaMetDex (l-asparaginase, methotrexate, and dexamethasone), have been highlighted as promising treatment regimens for newly diagnosed advanced-stage or relapsed/refractory ENKTL. However, some patients with stage III/IV ENKTL still experienced treatment failure following these l-asparaginase-containing regimens because of significant treatment-related toxicity [5, 7]. It is necessary to further optimize l-asparaginase-based regimen to circumvent these obstacles.
Gemcitabine has demonstrated substantial anticancer activity against NK cell lymphoma, possibly due to elevated expression of activating enzymes including deoxycytidine kinase (dCK) or equilibrative nucleoside transporter 1 (ENT1) in NK cells [10]. Similarly, oxaliplatin, a third generation organoplatinum derivative, has significant cytoxicity towards chemoresistant lymphoma cells and a favorable safety profile. In addition, oxaliplatin has shown mechanistic synergy when combined with gemcitabine in several studies of lymphoma [11, 12]. Moreover, pegaspargase displays lower incidence of antiasparaginase antibodies and more prolonged asparaginase activity in comparison with native l-asparaginase [13]. Therefore, several studies have reported encouraging results of the combination of gemcitabine, pegaspargase and oxaliplatin-based regimen for patients with stage I/II or relapsed/refractory ENKTL, such as GELOX (gemcitabine, pegaspargase and oxalipaltin) [2] and DDGP (gemcitabine, pegaspargase, cisplatin and dexamethasone) [6]. However, there are few studies available describing the efficacy of gemcitabine, pegaspargase and oxalipaltin combinations in newly diagnosed patients with stage III/IV ENKTL. This retrospective study presented a cohort of newly diagnosed advanced-stage ENKTL who received the Peg-GemOD regimen, i.e. pegaspargase combined with gemicitabine, oxaliplatin and dexamethasone as first-line therapy. It might provide rationale for extended clinical trials for pegaspargase and gemicitabine-based combinations in the treatment of advanced-stage ENKTL patients, and reinforce some important concepts in the treatment strategies of ENKTL in general.
Results from this retrospective study evaluating the response to treatment indicated that the Peg-GemOD regimen yielded an ORR of 67 % with 28 % CR rate, which was encouraging taking into account poor prognostic features of this advanced-stage patient population. Formal comparisons of response rate of Peg-GemOD with other commonly employed l-asparaginase or pegaspargase and gemicitabine-containing chemotherapy are problematic due to potential imbalance in patient selection. With respect to stage III/IV ENKTL, the observed response rate of the Peg-GemOD regimen was superior to those with traditional pegaspargase-based chemotherapy such as pegaspargase plus CHOP, EPOCH or GEMOX [14]. It was noteworthy that 8 of 11 patients with stage III ENKTL achieved CR or PR, which compared favourably to those with stage IV ENKTL. Moreover, patients with primary involved site other than upper aerodigestive tract tended to exhibit a poor response, which indicated that stage III/IV non-upper aerodigestive tract ENKTL was a unique subset and treatment strategies might be explored separately. Accordingly, the Peg-GemOD regimen appears to be effective for selected advanced-stage ENKTL patients, and further investigations are needed to study the selection and eligibility criteria for Peg-GemOD regimen. Furthermore, one patient with PR after 3 cycles converted to CR after a further three cycles of treatment, suggesting that some advanced-stage ENKTL patients might respond slowly. Therefore, treatment should not be abandoned for patients who do not seem to respond well at three cycles, so long as there is no significant toxicity to contraindicate continuation of treatment.
With the survival analysis, the median OS and PFS of these patients were 10 and 8.5 months, respectively. It was not surprising that responders after 6 courses of therapy had significantly longer OS and PFS than non-responders. In a sense, obtaining a good therapeutic response by induction chemotherapy is a prerequisite to a better prognosis in patients with advanced-stage ENKTL. However, it must be pointed out that there were only three patients with a CR showing durable responses. The high proportion of patients who progressed after achieving an initial response to 3 cycles of chemotherapy reflects the aggressive nature of advanced-stage ENKTL and suggests that more intensive consolidative treatment such as autologous or allogenenic hematopoietic stem cell transplantation may be appropriate for those transplant eligible CR patients with a curative intent [15–17]. Attempts to improve outcome in patients with advanced-stage ENKTL in remission have included studies of high-dose therapy (HDT) supported by autologous stem cell transplantation (ASCT), allogeneic hematopoietic stem cell transplantation (HSCT) and novel treatment modalities. Although some retrospective analyses of relatively small cohorts suggest a role of HDT–ASCT or allogeneic HSCT in the management of patients with stage III/IV ENKTL who have achieved CR with modern regimens, the optimal conditioning regimen, source of stem cell, and strategies of reducing transplant-related mortality (TRM) remains to be defined. We have recommended patients with advanced-stage or relapsed ENKTL who have achieved CR to proceed to HDT–ASCT or allogeneic HSCT if matched donor is available. However, some patients declined due to personal preference, concerns with TRM or financial issues, which led to only one patient who eventually had HDT–ASCT in this study. Continued efforts should be devoted to define the subset of patients with advanced-stage ENKTL who may benefit most from HDT–ASCT or allogeneic HSCT.
Plasma EBV-DNA level was shown to be associated with OS and PFS of stage III/IV ENKTL patients in our study. EBV plays an important role in lymphomagenesis of ENKTL [18]. Several studies have shown that plasma EBV-DNA level is a sensitive surrogate biomarker for ENKTL tumor load. Persistently undetectable plasma EBV-DNA in ENKTL patients following treatment is correlated with superior survival [19, 20]. In this study, patients who achieved undetectable plasma EBV-DNA following treatment with the Peg-GemOD regimen were still alive and maintained CR status, which indicates that plasma EBV-DNA level is an independent predictor for OS and PFS in advanced-stage ENKTL. There were three patients who achieved CR and maintained durable responses. However, insufficient follow-up time and sample size restricted the accurate evaluation of OS and PFS. Further study will collect more patients with sufficient follow-up.
As to adverse events, this retrospective study showed that the Peg-GemOD regimen had moderate toxicity and was generally tolerable. Myelosuppression (grade 3/4 neutropenia and thrombocytopenia) was the main toxicity leading to dose reduction and treatment delay. However, hematological toxicity was manageable with growth factor support, empirical antimicrobial treatment or blood component transfusions. Treatment-related serious infections were uncommon. Other non-hematological toxicities mainly related to pegaspargase could be well controlled with supportive measures. Importantly there were rare life-threatening complications. Moreover, patients with stage III/IV ENKTL presented with worse ECOG PS and marrow involvement, which might complicate the interpretation of toxicity profile of the Peg-GemOD regimen. Therefore, Peg-GemOD regimen, to a large extent, is a well-tolerated chemotherapy with an acceptable level of toxicity for advanced ENKTL.
In conclusion, this retrospective study suggests that the Peg-GemOD regimen is a promising induction chemotherapy for patients with newly diagnosed advanced-stage ENKTL. From patient treatment point of view, Peg-GemOD regimen with highly effective and acceptable toxicity may provide a favorable treatment option for stage III/IV ENKTL. On a broader perspective, this study serves as a platform for the design of future clinical trials exploring novel therapeutic strategies based on pegaspargase–gemcitabine–oxaliplatin combination in improving the long-term outcome of ENKTL patients.
Acknowledgments
The authors are grateful to Dr. Chun Wang and Dr. Yeh-Ching Linn for help in reviewing the manuscript. The authors are grateful to all members from the Hematology Department of Shanghai Ninth People’s Hospital for their continuous support and encouragement.
Compliance with Ethical Standards
Conflict of interest
All authors have no conflicts of interest. This work was supported by Science and Technology Commission of Shanghai Municipality (Grant No. 12ZR1416800, 13ZR1423800).
Ethical Standards
The study was approved by the Hospital Ethics Committee and conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Yi-yun Yao, Yong Tang and Yan Zhuang have contributed equally to this work.
References
- 1.Aozasa K, Takakuwa T, Hongyo T, et al. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008;87(2):110–117. doi: 10.1007/s12185-008-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119(2):348–355. doi: 10.1002/cncr.27752. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi M, Tobinai K, Oguchi M, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan clinical oncology group study JCOG0211. J Clin Oncol. 2012;30(32):4044–4046. doi: 10.1200/JCO.2012.45.6541. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76(11):2351–2356. doi: 10.1002/1097-0142(19951201)76:11<2351::AID-CNCR2820761125>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Jaccard A, Gachard N, Marin B, et al. GELA and GOELAMS Intergroup. Efficacy of l-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Li X, Chen C, Li X, Zhang L, et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. 2014;93(11):1889–1894. doi: 10.1007/s00277-014-2136-7. [DOI] [PubMed] [Google Scholar]
- 7.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 8.Ahn HK, Kim SJ, Hwang DW, et al. Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Investig New Drugs. 2013;31(2):469–472. doi: 10.1007/s10637-012-9889-4. [DOI] [PubMed] [Google Scholar]
- 9.Yao YY, Tang Y, Zhu Q, et al. Gemcitabine, oxaliplatin and dexamethasone as salvage treatment for elderly patients with refractory and relapsed peripheral T-cell lymphoma. Leuk Lymphoma. 2013;54(6):1194–1200. doi: 10.3109/10428194.2012.739286. [DOI] [PubMed] [Google Scholar]
- 10.Elnaggar M, Giovannetti E, Peters GJ. Molecular targets of gemcitabine action: rationale for development of novel drugs and drug combinations. Curr Pharm Des. 2012;18(19):2811–2829. doi: 10.2174/138161212800626175. [DOI] [PubMed] [Google Scholar]
- 11.Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica. 2013;98(11):1726–1731. doi: 10.3324/haematol.2013.090597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El GT, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18(8):1363–1368. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 13.Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–2033. doi: 10.1182/blood-2013-10-534347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen JY, Li M, Li X, et al. Efficacy and tolerance of pegaspargase-based chemotherapy in patients with nasal-type extranodal NK/T-cell lymphoma: a pilot study. Asian Pac J Cancer Prev. 2014;15(15):6275–6281. doi: 10.7314/APJCP.2014.15.15.6275. [DOI] [PubMed] [Google Scholar]
- 15.Kwong YL. High-dose chemotherapy and hematopoietic SCT in the management of natural killer-cell malignancies. Bone Marrow Transplant. 2009;44(11):709–714. doi: 10.1038/bmt.2009.239. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Bang SM, Lee J, et al. High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant. 2006;37(9):819–824. doi: 10.1038/sj.bmt.1705349. [DOI] [PubMed] [Google Scholar]
- 17.Ennishi D, Maeda Y, Fujii N, et al. Allogeneic hematopoietic stem cell transplantation for advanced extranodal natural killer/T-cell lymphoma, nasal type. Leuk Lymphoma. 2011;52(7):1255–1261. doi: 10.3109/10428194.2011.572322. [DOI] [PubMed] [Google Scholar]
- 18.Tomita Y, Ohsawa M, Mishiro Y, et al. Epstein-Barr virus in lymphoproliferative disease in the sino-nasal region: close association with CD56+ immunophenotype and polymorphic reticulosis morphology. Int J Cancer. 1997;70(1):9–13. doi: 10.1002/(SICI)1097-0215(19970106)70:1<9::AID-IJC2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Kimura H, Maeda Y, et al. Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res. 2012;18(15):4183–4190. doi: 10.1158/1078-0432.CCR-12-1064. [DOI] [PubMed] [Google Scholar]
- 20.Kwong YL, Pang AW, Leung AY, et al. Quantification of circulating Epstein-Barr virus DNA in NK/T-cell lymphoma treated with the SMILE protocol: diagnostic and prognostic significance. Leukemia. 2014;28(4):865–870. doi: 10.1038/leu.2013.212. [DOI] [PubMed] [Google Scholar]