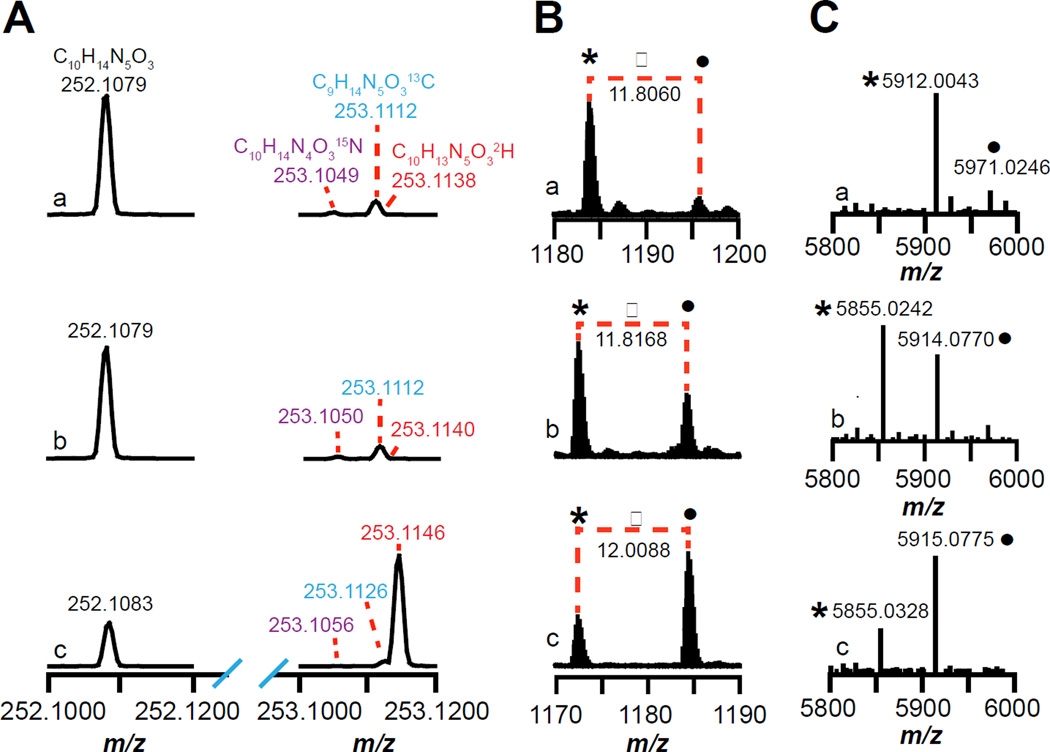
Figure 2.
Mass spectra of (A) dAdo or (B and C) SkfA isolated from reactions containing (a) wild-type, (b) M12[β-D3]A–, or (c) M12[U-D4]A–SkfA. (A) The mass spectra show the monoisotopic (m/z = 252.1079 – 252.1083) and the corresponding 15N (m/z = 253.1049 – 253.1056), 13C (m/z = 253.1112 – 253.1126), and 2H isotope peaks (m/z = 253.1138 – 253.1146) of dAdo. (B) Mass spectra of SkfA peptides zooming in on the +5 charge state (see Fig S4a–c for the full mass spectra). The peak indicated by • corresponds to SkfA with three carbamidomethylated cysteines, whereas the * corresponds to SkfA containing one thioether crosslink and two carbamidomethylated cysteines. (C) The deconvoluted mass spectra generated using the Xtract software (Thermo Fisher) from the full mass spectra shown in Fig S4a–c.