Fig. 6.
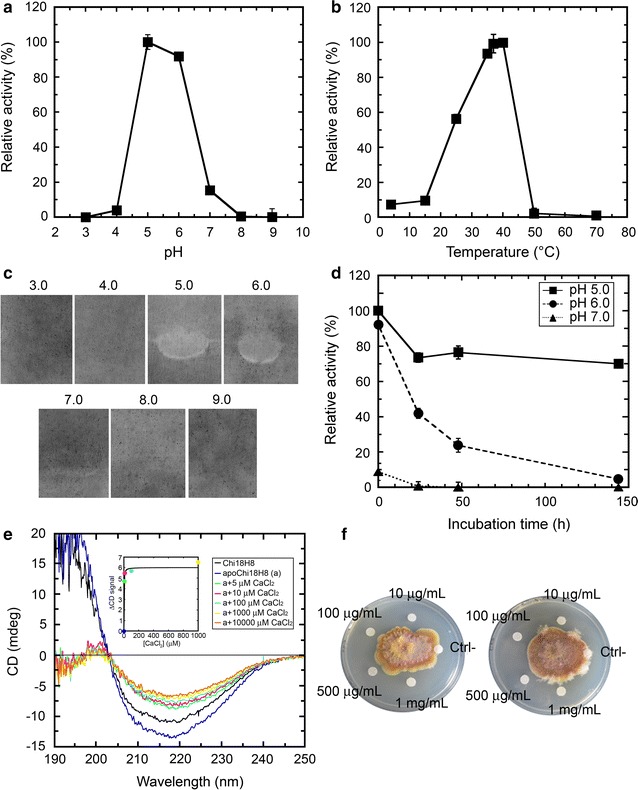
Characterization of Chi18H8 purified from IBs. a pH profile of Chi18H8 activity, using 4-MU-(GlcNAc)2 as substrate. b Temperature influence of Chi18H8 activity on 4-MU-(GlcNAc)2 measured in 100 mM sodium acetate buffer, pH 5.0. c Effect of pH on Chi18H8 activity on CM-chitin-RBV (zymograms in semi-denaturing conditions). d Time course of stability of Chi18H8 at different pHs. The enzyme was incubated in 100 mM sodium acetate pH 5.0, sodium phosphate pH 6.0 or sodium phosphate pH 7.0 for up to 144 h at 30 °C. At various time intervals, samples were withdrawn and the residual chitinolytic activity was fluorimetrically measured on 4-MU-(GlcNAc)2 under standard conditions. In panels a, b and d, the activity is expressed as a percentage of the maximum activity (set as 100%) and the values represent the mean of three experiments (mean ± standard error). e Circular dichroism (CD) far-UV spectra of the purified chitinase (indicated as ‘Chi18H8’), of the same preparation following EDTA addition and dialysis to eliminate divalent ions (‘apoChi18H8’), and after adding increasing concentrations of CaCl2. The concentration of Chi18H8 was 0.2 mg/mL in 10 mM HEPES pH 5.6. In the inset, the ΔCD signals at 219 nm vs. CaCl2 concentration used to extrapolate the dissociation constant Kd, are reported. f Antifungal activity of Chi18H8 repressing Fusarium graminearum growth on PDA plates. Reduction in radial growth of the centrally inoculated fungi was evident around discs embedded of Chi18H8 at 1000, 500, 100 or 10 µg/mL concentration. No growth inhibition was observed in the negative control (Ctrl−), i.e. boiled Chi18H8. The image shows two replicates (out of three)
