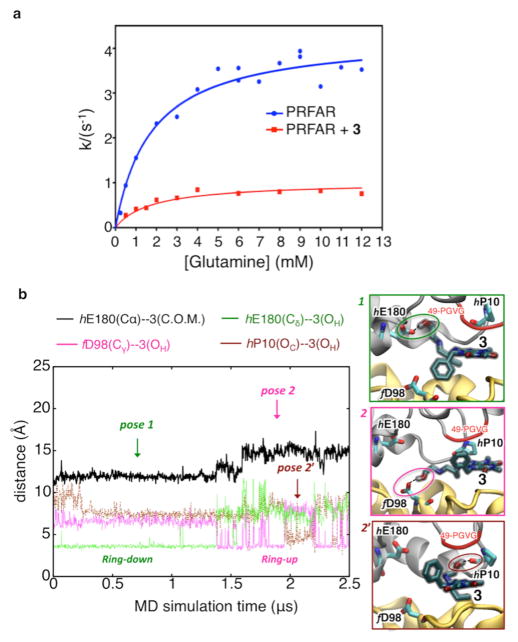
Figure 5.
Suppression of PRFAR-enhanced glutaminase activity upon binding of 3. (a) Inhibition of glutaminase activity of the PRFAR–IGPS binary complex (blue) by 3 (red) as measured by kinetic assays. Nonlinear least-squares fitting of a noncompetitive binding model yields a Ki of 0.9 ± 0.07 mM. (b) Microsecond MD simulations of inhibitor binding. Binding of 3 to the PRFAR-bound IGPS binary complex is monitored along a representative 2.5 μs MD trajectory, showing two main poses (1, 2, and a transient pose 2′) characterized by specific H-bonds (highlighted with dotted lines and colored circles) and hydrophobic contacts between the benzyl functional group of 3 and HisF (ring down) or alternatively HisH (ring up).