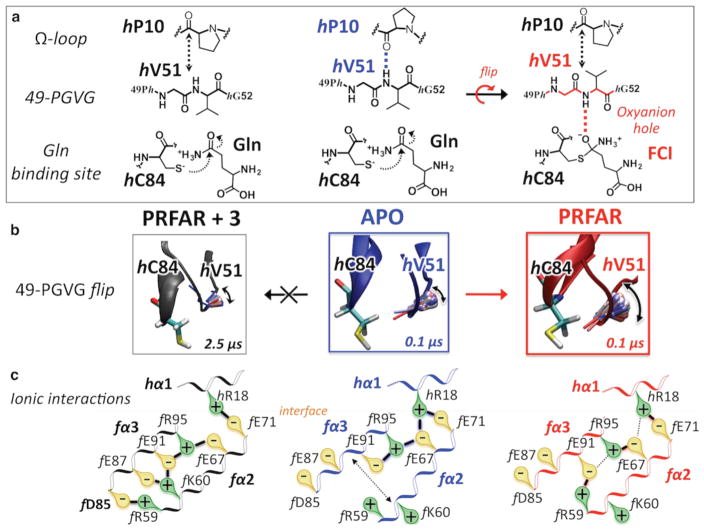
Figure 6.
Mechanism of IGPS allosteric signal disruption by inhibitor binding. Changes in ionic interactions among the fα2, fα3, and hα1 helices (bottom row) in the apo IGPS complex (blue, center column) induced by PRFAR binding (red, right column) and subsequently altered by inhibitor binding (black, left column). Disruption of the allosteric signal launched by PRFAR manifested as hampered oxyanion strand flip, which is initiated on the submicrosecond time scale in the binary complex while absent in the 3–IGPS ternary complex (middle row). The flip of the 49-PGVG strand is crucial for glutaminase activity because it allows stabilization of a four-center intermediate (FCI) in the oxyanion hole (top row).