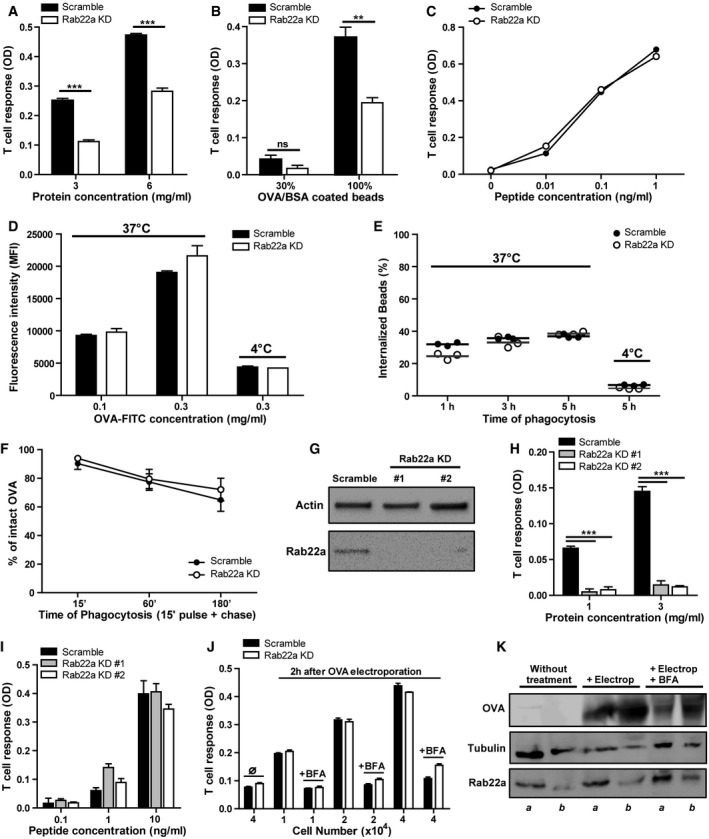
Figure 3. Rab22a controls cross‐presentation by DCs without affecting antigen internalization, phagosomal degradation, or endogenous MHC‐I presentation.
-
A–CThe cross‐presentation ability after incubation with (A) soluble OVA, (B) OVA/BSA‐coated beads, and (C) the SIINFEKL control peptide at the indicated concentrations by Scramble and Rab22a KD JAWS‐II DCs was evaluated with the B3Z hybridoma. Data represent mean ± SEM of triplicate values and are representative of three independent experiments. (A) ***P = 0.0001 and (B) P = 0.1432 (ns); **P = 0.0044. The two‐tailed Student's unpaired t‐test was performed.
-
D, EEvaluation of endocytosis and phagocytosis in Scramble and Rab22a KD JAWS‐II DCs. (D) The endocytosis of fluorescent OVA after 1 h of internalization and (E) the phagocytosis of 3‐μm fluorescent LB at different times of internalization were assessed by FACS analysis. The antigen internalization was conducted at 37°C for effective uptake and at 4°C as negative control. In (D), data represent mean ± SEM of triplicate values and are representative of three independent experiments.
-
FThe kinetics of OVA degradation, as percentage of proteases inhibitors, in isolated phagosomes at the indicated time periods post‐internalization from Scramble and Rab22a KD JAWS‐II DCs was assessed by FACS analysis. Data represent mean ± SEM of three independent experiments.
-
GImmunoblotting of Rab22a and Actin in BMDCs infected with lentiviruses encoding a random sequence (Scramble) and two shRNA specific for silencing Rab22a (Rab22a KD #1 and #2).
-
H, IThe cross‐presentation capacity after the incubation with (H) soluble and (I) the SIINFEKL control peptide at the indicated concentrations by Scramble, Rab22a KD #1, and Rab22a KD #2 BMDCs was evaluated as described before for JAWS‐II DCs. Data represent mean ± SEM of triplicate values and are representative of two independent experiments. ***P = 0.0001. The two‐tailed Student's unpaired t‐test was performed.
-
JSoluble OVA was electroporated into the cytosol of Scramble and Rab22a KD JAWS‐II DCs, and T‐cell activation was determined 2 h later with the B3Z hybridoma. To control endogenous MHC‐I antigen presentation specificity, DCs were also treated with brefeldin A (BFA). The use of this drug markedly reduced CD8+ T‐cell response to similar levels obtained by DCs without any antigen (∅). Data represent mean ± SEM of triplicate values and are representative of three independent experiments.
-
KImmunoblotting showing the amount of OVA incorporated by Scramble (a) and Rab22a KD (b) JAWS‐II DCs after electroporation and BFA treatment.
Source data are available online for this figure.
