Figure EV5. The Mdm12–Mdm34 interaction might be mediated through the N‐terminus and the N‐terminus of the SMP domain in Mmm1 might resemble that in E‐SYT2.
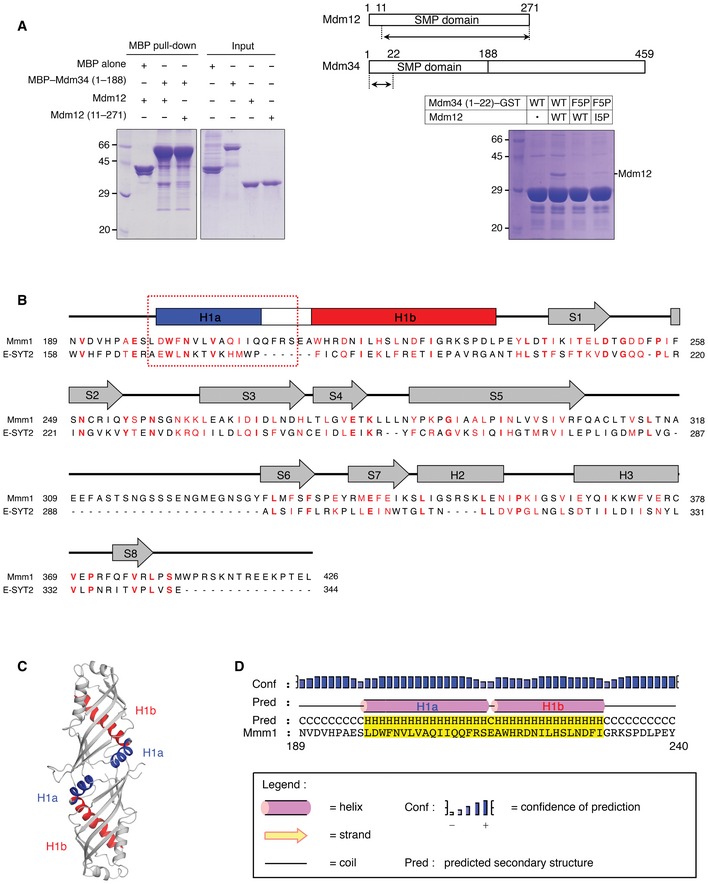
- MBP pull‐down experiment (left) showing that the SMP domain of Mdm34 interacts with full‐length Mdm12 but not with N‐terminus‐truncated Mdm12 (residues 11–271). GST pull‐down experiment (right) indicates that the N‐terminal fragment (residues 1–22) of Mdm34 can interact with the Mdm12. The constructs used in the experiments are shown above.
- Sequence alignment of SMP domains in Mmm1 and E‐SYT2. The relatively conserved sequences are highlighted in red. The secondary structure elements are indicated above the sequences with helices and strands as arrows and cylinders, respectively, based on the crystal structure of E‐SYT2 29. The N‐terminus of Mmm1 that is predicted to form an α‐helix (H1a) and make a twofold interface for Mmm1 self‐association is indicated by a red square 39. The sequences corresponding to H1a are highly conserved in E‐SYT2 and Mmm1.
- Ribbon diagram of SMP domain of E‐SYT2 highlighting the twofold interface. The color scheme is the same as in (A).
- Secondary structure prediction of the N‐terminus of Mmm1 comprising residues 189–240. Explanations of the different symbols are given in the box.
