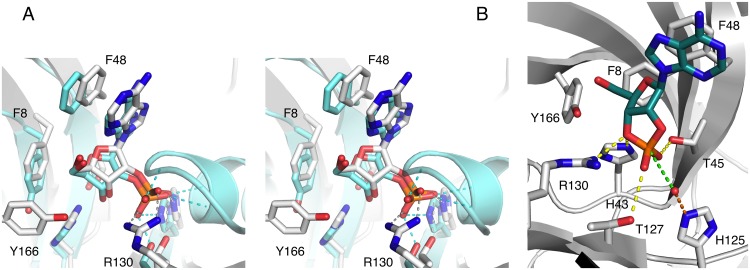
Fig 3. Product and substrate binding.
A. Comparison of the binding modes of 2′-AMP in LigT (white) and mouse CNPase (blue). Interactions of the phosphate are indicated by dashed lines; note the binding of the phosphate by Arg130 in LigT, while the phosphate is coordinated to the helix α7 N terminus in CNPase. The other interactions are essentially identical. B. Modelling of the substrate complex of LigT. 2′,3′-cAMP and the nucleophilic water were modeled into the LigT active site based on CNPase structures and the 2′-AMP binding mode to LigT. Polar interactions are shown by yellow dashed lines, and the activation of the water molecule by His125 by an orange dashed line. The direction of nucleophilic attack is shown by the green dashed line. His43 (behind) donates a proton to the leaving group, the ribose 3′-oxygen.