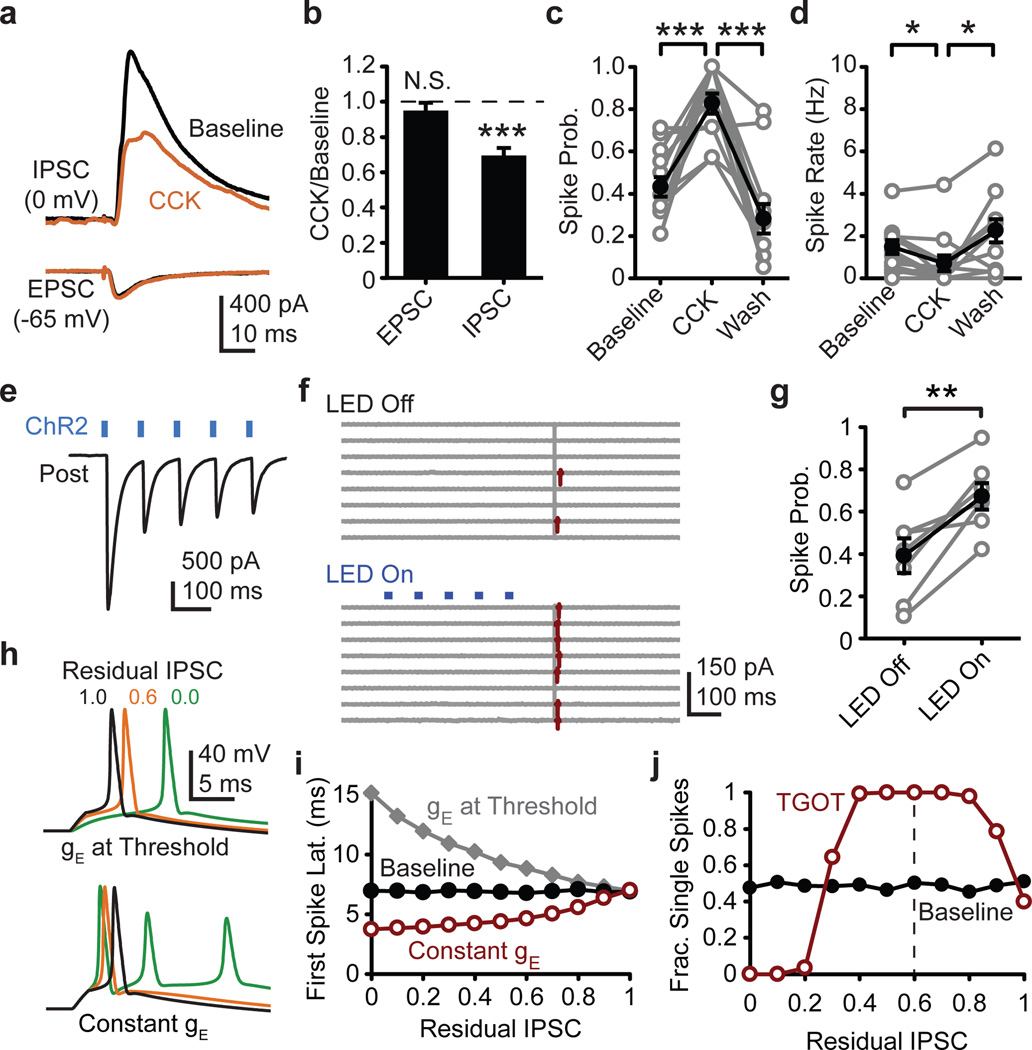
Figure 4. Generalization to other brain states and modulators.
a, CCK (200 nM) influence on average evoked disynaptic IPSC from one pyramidal cell and monosynaptic EPSC from a different pyramidal cell. b, Normalized group data for evoked EPSC (N=6 cells) and disynaptic IPSC (N=6 cells). c, Evoked spike probability (N=14 cells) and d, spontaneous firing rate (N=14 cells) in cell-attached recordings as influenced by CCK. e, Exemplar ChR2-evoked IPSCs recorded in a CA1 pyramidal neuron from a PV-cre mouse injected with double-floxed AAV-ChR2. f, Cell-attached recording from mouse CA1 pyramidal neuron. Control (LED off) and ChR2 stimulation (LED on) sweeps interleaved during recording, but grouped for presentation. g, ChR2 influence on cell-attached spike probability in the subset of mouse pyramidal neurons in which latency and jitter indicated a minimal monosynaptic inhibition (see methods). h, Computer simulated exemplar traces in which IPSC conductance (gIPSC) is reduced and EPSC conductance (gEPSC) is either lowered to maintain ~50% chance of spiking (top) or gEPSC is held constant (bottom). i, Residual IPSC influence on simulated spike latency with gEPSC held constant (red) or reduced to maintain ~50% spike probability (gray). Panels h and i generated in absence of spontaneous IPSCs to isolate feed-forward IPSC contribution to evoked spike timing. j, Residual IPSC influence on probability of eliciting exactly one spike (gEPSC held constant). Paired two-tailed t-test. *, P<0.05; **, P<0.01; ***, P<0.001. Error bars S.E.M.