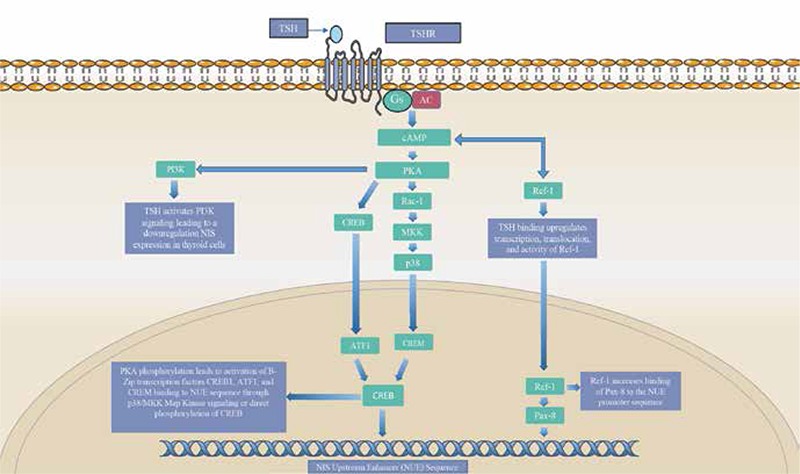
Figure 6. Sodium iodide symporter gene signaling *The sodium Iodide symporter is a transmembrane protein that plays a crucial role in the uptake of iodine by thyroid cells. The SCL5A5 gene is regulated by the activity of TSH on the sodium iodide symporter upstream enhancer sequence (NUE). In this pathway TSH binding to TSHR stimulates an increase in cyclic AMP (cAMP) levels in the cell. Elevated levels of cAMP lead to the activation of protein kinase-A (PKA) dependent p38/MAPK and PKA-cAMP-response element binding protein (CREB) pathways. The p38/MAPK pathway leads to the recruitment of basic-leucine zipper transcription factors ATF1, and cAMP-response modulator to the nucleus. In the nucleus these transcription factors bind to the cAMP-response element binding sites in the NUE and activating the SCL5A5 gene. PKA can also directly phosphorylate the cAMP-CREB to directly regulate cAMP responsive genes such as TSHR, and SCL5A5. Full activation of the SCL5A5 gene is not reached until the PAX-8 transcription factor bindings to the NUE promoter site. TSH signaling upregulates the transcription and nuclear translocation of the oxidative stress factor Ref-1. Ref-1 activates and recruits PAX-8 to bind to the NUE promoter site, leading to full SCL5A5 gene activity. Inhibition of SCL5A5 activity can also occur due to the elevated levels of cAMP. These elevated levels can trigger activation of the PI3K signaling pathway leading to the downregulation of the SLC5A5 gene.