Abstract
Chronic ethanol intake impairs liver regeneration through a system-wide alteration in the regulatory networks driving the response to injury. Our study focused on the initial phase of response to 2/3rd partial hepatectomy (PHx) to investigate how adaptation to chronic ethanol intake affects the genome-wide binding profiles of the transcription factors C/EBP-β and C/EBP-α. These factors participate in complementary and often opposing functions for maintaining cellular differentiation, regulating metabolism, and governing cell growth during liver regeneration. We analyzed ChIP-seq data with a comparative pattern count (COMPACT) analysis, which exhaustively enumerates temporal patterns of discretized binding profiles to identify dominant as well as subtle patterns that may not be apparent from conventional clustering analyses. We found that adaptation to chronic ethanol intake significantly alters the genome-wide binding profile of C/EBP-β and C/EBP-α before and following PHx. A subset of these ethanol-induced changes include C/EBP-β binding to promoters of genes involved in the profibrogenic transforming growth factor-β pathway, and both C/EBP-β and C/EBP-α binding to promoters of genes involved in the cell cycle, apoptosis, homeostasis, and metabolic processes. The shift in C/EBP binding loci, coupled with an ethanol-induced increase in C/EBP-β binding at 6 h post-resection, indicates that ethanol adaptation may change both the amount and nature of C/EBP binding postresection. Taken together, our results suggest that chronic ethanol consumption leads to a spatially and temporally reorganized activity at many genomic loci, resulting in a shift in the dynamic balance and coordination of cellular processes underlying regenerative response.
Keywords: liver regeneration, ethanol, partial hepatectomy, chromatin immunoprecipitation, ChIP-seq, pattern analysis, C/EBP-β, C/EBP-α
liver regeneration is the process through which mature hepatocytes enter into a program of compensatory hyperplasia to replace tissue mass lost through mechanical or toxic damage. Clinical interventions rely on this highly coordinated cellular response following tissue resection for treatment of hepatocellular carcinoma or to allow for live liver transplant, with regeneration induced in both donor and recipient. In the laboratory setting, researchers induce regeneration in rodents and other mammals using a partial hepatectomy (PHx) procedure. During PHx, up to 70% of the liver mass is removed through surgical resection, and the remaining tissue regenerates to replace the lost mass (15, 43). Following PHx, a coordinated tissue response occurs involving multiple cell types and signaling factors to induce hepatocytes to re-enter the cell cycle (23, 33, 41). Immediate early signals, including inflammatory cytokines and Wnt-family molecules, trigger intra- and intercellular processes through which nonparenchymal cells secrete factors necessary for the entry of hepatocytes into the cell cycle (43). These factors trigger regulation of transcriptional programs in hepatocytes, promoting the entry into the cell cycle.
One set of important regulators of regeneration-associated hepatocyte transcription programs are the CCAAT/enhancer-binding transcription factors, C/EBP-α and C/EBP-β (4, 47, 50, 51). They belong to the basic-region leucine zipper family (bZIP) and bind to the same core DNA sequences as homo- or heterodimers (48). The C/EBPs have been shown to have a functional role during the initiation of regeneration as well as during the termination phase (12, 13, 61, 63). C/EBP-β is upregulated during liver regeneration and contributes to the induction of hepatocyte replication (12, 16, 19). In contrast, C/EBP-α expression and DNA binding activity are at high levels in the resting liver, but levels decrease within the first few hours following injury (12, 18, 44, 57). Furthermore, the established role of C/EBP-α in cell cycle arrest and terminal differentiation (31) suggests that this factor could be acting as an antiproliferative factor post-PHx. In contrast, C/EBP-β knockout mice show reduced DNA synthesis leading to inhibition of regeneration (19).
Previous studies focused on the opposing roles of C/EBP-α and C/EBP-β in the context of liver repair and regeneration (18). A prominent feature is the switch-like behavior of these two transcription factor family members that is expected to play a major role in shaping the regeneration profile after resection or other injury. C/EBP-α activity decreases at the same time that C/EBP-β activity increases during the initiation phase of regeneration, resulting in an approximate sevenfold increase in the C/EBP-β-to-C/EBP-α ratio and coinciding with the timing of the onset of hepatocyte proliferation (17). Further supporting the notion that the C/EBP-β-to-C/EBP-α ratio is important for regeneration, a recent study has shown that when nonfunctional C/EBP-α was knocked-in to mice, liver regeneration was sustained through the time period when termination normally occurs and liver mass was increased to ~130% of initial liver size (30). Recently, the temporal binding patterns of C/EBP-α and C/EBP-β were mapped using ChIP-seq in regenerating mouse livers from 0 to 168 h post-PHx (28). In the regenerating mouse liver, C/EBP-α and C/EBP-β bound to three prevalent clusters of genes: two clusters with strong C/EBP-β binding and one cluster with coordinated C/EBP-α and C/EBP-β binding. These clusters contained genes associated with inflammatory response, multiple metabolic pathways, and the cell cycle (among others), indicating that C/EBP-α and C/EBP-β binding may play an important role in regulating multiple functional responses during the course of liver regeneration.
Adaptation to chronic ethanol intake is known to interrupt the damage response pathways of the liver, thus impairing its regenerative capacity (34, 60, 67). Rats subjected to PHx following chronic alcohol consumption had lower levels of hepatocytes entering the cell cycle and a suppression of overall mass recovery (14). Chronic alcohol consumption has also been shown to affect C/EBP activity. We hypothesize that chronic alcohol consumption may inhibit liver regeneration in part through deregulating the dynamic profiles of C/EBP-α and C/EBP-β binding. In humans, the progression from alcoholic steatosis to alcoholic hepatitis is associated with a nearly twofold decrease in C/EBP-β mRNA levels (53). Ethanol intake for 5–10 wk has been shown to elevate C/EBP-β expression by 1.4-fold in mice (6). In rats, alcohol consumption decreases the DNA binding activity of C/EBP-α, but not that of C/EBP-β, when the animals were fed 20% ethanol via drinking water for 1–2 wk (22). These effects may be mediated through a dysregulation in the IL-6 and JAK-STAT signal transduction pathways. Additionally, recent work linked such an alcohol-mediated suppression of C/EBP-α to the expression of hypoxia-inducible factor-1α (HIF-1α) (1). These results suggest that C/EBP-α response to alcohol consumption may be dynamically altered in the regenerating liver, as the hepatocytes are exposed to oxygen-rich as well as oxygen-poor microenvironments, depending on the lobular location.
In the present study, we investigated the dynamic, genome-wide binding patterns of C/EBP-α and C/EBP-β following PHx in chronic alcohol-fed rats and controls. We employed a novel COMparative PAttern CounTs (COMPACT) analysis to interrogate the effects of chronic alcohol consumption on C/EBP-α and C/EBP-β regulation of liver regeneration. Our results identified a coordinated switch-like regulation of C/EBP-α and C/EBP-β promoter binding in the liver during the initial response to partial hepatectomy, which was disrupted in the ethanol-adapted animals.
MATERIALS AND METHODS
Experimental Protocol
All animal studies were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. Adult Sprague-Dawley rats were held in a climate-controlled, 12 h day/night cycle in accordance with accepted animal handling practices. Animals were fed using the Lieber-DeCarli pair-feeding model (38) in which rats were fed a nutritionally adequate liquid diet containing 36% of total calories derived from ethanol, 11% from carbohydrate, 18% from protein, and 35% from fat for 5 wk (Ethanol group), with the pair-fed calorie-matched littermate controls receiving liquid diets in which ethanol calories were replaced by maltose dextran (Carbohydrate group). Rats (275–350 g) were anesthetized and subjected to 2/3rd PHx by surgical removal of left lateral and median lobes as previously described (10). The remnant liver was allowed to regenerate, and the liver samples were harvested at 6 h post-PHx. The excised liver samples at t = 0 served as within-animal controls. Collected liver samples were flash-frozen in liquid nitrogen-cooled aluminum clamps for preparation of tissue lysates.
Chromatin Immunoprecipitation Followed by Sequencing (ChIP-seq)
Approximately 50 µg of minced liver tissue was fixed for 10 min with 1% formaldehyde to cross-link DNA and chromatin-binding proteins such as transcription factors to ensure coimmunoprecipitation (as per standard protocol using a Magna ChIP G kit obtained from Millipore, Billerica, MA). Cross-linked chromatin was neutralized by addition of excess glycine to quench unreacted formaldehyde. Chromatin was sheared by sonication to generate fragments of 200–1,000 bp size. The fragment size range was confirmed by a 1% agarose gel electrophoresis. C/EBP-α and C/EBP-β chromatin immunoprecipitation (ChIP) were performed using 1 µg cross-linked chromatin and subsequent immunoprecipitation with 2 µg antibody from Santa Cruz Biotechnology (C/EBP-α: Cat. #sc-61X and C/EBP-β: Cat. #sc-150X). The immunoselection was performed using the ChIP grade antibody in combination with protein G-conjugated solid support matrix magnetic beads to enrich for the specific DNA-protein complex of interest.
ChIP was followed by ultrahigh-throughput sequencing using the ABI SOLiD ChIP-seq platform to detect C/EBP-β and C/EBP-α binding targets in liver samples obtained at 6 h following PHx. SOLiD reads were aligned to the reference genome (Rn4) using Bioscope 1.2 with default parameters (seed of 25 bases with 2 mismatches for 50 base-pair reads). The alignment algorithm used a seed-and-extend approach similar to BLAST. The alignment was extended in both directions with a mismatch penalty of −2 and a matching score of +1, and the alignment with the highest score was reported.
Peak Detection
One of the most critical aspects of a ChIP-seq analysis is the identification of peak regions. We obtained data from four biological replicates in the time series ChIP-seq experiments for C/EBP-β and C/EBP-α for each diet group, except for the ChIP-seq experiments on C/EBP-α binding in the ethanol group at 6 h post-PHx, where we obtained data from three biological replicates. For C/EBP-β, the total number of reads obtained from the sequence analyzer ranged from 25 million to 138 million. In the case of C/EBP-α, the total number of reads obtained from the sequence analyzer ranged from 9 million to 59 million. For C/EBP-β, we used three different peak finding algorithms (Cisgenome, PARTEK, MACS) to identify enriched genomic loci, known as peaks (29, 68). For Cisgenome, (bin size = 40, half window size = 2, read extension length = 200, maxgap = 100) we used a fold-change cut-off of 5.0. There was some amount of variability between the numbers of peaks obtained by these algorithms. In the case of C/EBP-β, overlapping genomic loci in two or more tools were retained as a peak. In the case of C/EBP-α, peaks were identified using Cisgenome (bin size = 40, half window size = 2, read extension length = 200, maxgap = 100) with a fold-change cut-off of 2.8. Subsequently, the peaks were annotated for various features using the Bioconductor package ChIPpeakAnno (69). Annotations include, for each peak, the gene with the nearest transcription start site (TSS) or overlapping gene and annotation for other genomic features such as peak location, region width, strand, gene ID, gene start, gene end, location, and distance.
COMPACT Analysis
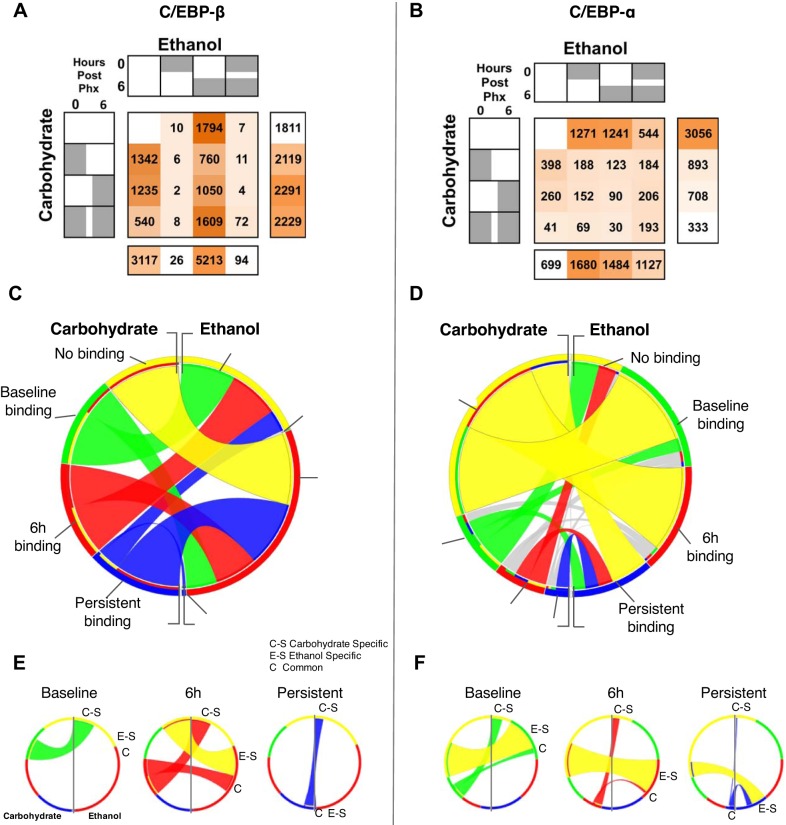
We were interested in exploring the differences in patterns of binding loci between 1) ethanol vs. control diet in C/EBP-β and C/EBP-α and 2) C/EBP-β vs. C/EBP-α in ethanol and control diet groups, respectively. As an initial step, we created all possible combinations of digitized dynamic patterns between the respective groups using an unbiased data-driven approach termed COMPACT analysis. We represented the number of promoters corresponding to each of the 16 combinational patterns (4 patterns × 2 dietary groups) into a table (Fig. 1, A and B). This gave us a systems-level view of the evolution of the C/EBP-β and C/EBP-α binding landscape in the adapted and post-PHx states. To better visualize the control vs. ethanol comparison, we used a chord diagram representation using the CIRCOS v0.67 software, separately for C/EBP and C/EBP-α (35). In this scheme, arcs of appropriately scaled length represented the counts for patterns within each diet group (Fig. 1, C and D). Ribbons with different colors represent dynamic patterns, with the width of ribbons proportional to the number of genes. Thus, each of the rows and columns of the COMPACT matrix is represented as a segment, and the size of the ribbon connecting them encode the common number of genes associated with the pair. Relative row, column, and overall total size of each segment are shown in outer circular patches.
Fig. 1.
Comparative pattern count (COMPACT) analysis of dynamic C/EBP binding to putative target genes. A: C/EBP-β dynamic binding patterns postpartial hepatectomy (post-PHx) in ethanol-fed rats and controls. This COMPACT representation shows numbers of genes bound in each dynamic binding pattern. B: C/EBP-α dynamic binding patterns post-PHx in ethanol-fed rats and controls. C: chord diagram representation of dynamic C/EBP-β binding. D: chord diagram representation of dynamic C/EBP-α binding. E: select examples of ethanol-specific, common, and carbohydrate-specific genes bound by C/EBP-β at baseline, at 6 h post-PHx, and with persistent binding. F: select examples of ethanol-specific, common, and carbohydrate-specific genes bound by C/EBP-α at baseline, at 6 h post-PHx, and with persistent binding.
We further expanded this matrix to include both transcription factors in one matrix with 256 combinatorial patterns (16 patterns × 2 dietary groups). Each row and column of the COMPACT matrix consisted of the number of genes corresponding to a dynamic pattern. This approach helped us to systematically explore the gene sets and to narrow them down to certain subsets of interest for detailed downstream analysis.
Pathway Analysis
Relevant gene subsets identified using COMPACT analysis were analyzed for biological functions using DAVID v6.7 (Database for Annotation, Visualization and Integrated Discovery) (24). DAVID provides a comprehensive set of functional annotation tools to identify biological functions behind a large list of genes, by which we identified enriched biological themes, particularly gene ontology (GO) terms. We used an unadjusted P value threshold of 0.05 to filter the pathway list to reduce the pathways to the most significant ones.
Transcription Factor Binding Site Analysis for Motifs
Transcription factor binding motif identification was performed using DME (discriminating motif enumerator) (54), a de novo motif discovery program based on an enumerative algorithm. DME identifies optimal motifs from a discrete space of matrices with a specific lower bound on information content and is suited for analyzing large data sets. String lengths ranging from 8 to 14 nucleotides were used to scan for motifs. Following DME analysis, we employed STAMP, a motif clustering program, to scan the DME-identified de novo motifs against a known transcription factor binding database (39). STAMP analysis returned high scoring motifs corresponding to known transcription factor binding sites, which were visualized as logos.
RESULTS
We examined the total number of binding peaks for C/EBP-α and C/EBP-β as a correlate of the transcription factor activity (3 or 4 biological replicates for C/EBP-α and C/EBP-β, for each dietary group – ethanol and carbohydrate control, and for each time point – baseline and 6 h post-PHx). We employed the ChIPpeakAnno tool to identify the candidate target genes with TSS) that were at the shortest distance from the binding peaks. In those targets bound only following chronic ethanol adaptation (Ethanol specific), there was little change in the number of C/EBP-α bound genes, while the number of C/EBP-β bound target genes increased (Table 1, Ethanol specific). In the genes bound only in the control group (Carbohydrate specific), as well as in the genes bound in both groups (Common), PHx did not lead to a significant change in the number of C/EBP-α bound genes (Table 1, Carbohydrate specific and Common). C/EBP-β, on the other hand, showed an increase in the number of bound genes in both ethanol-fed and control rats (Table 1, Common) and a decrease in the number of bound genes only in the control rats (Table 1, Carbohydrate specific). Our results point to a differential effect of ethanol on the number of C/EBP-β bound genes, but not on C/EBP-α bound genes, after PHx. However, a detailed analysis of the specific genes and corresponding functions is needed before these differences can be interpreted for putative mechanistic contribution to the ethanol-mediated impairment of liver regeneration.
Table 1.
Number of genes bound only in the control group (Carbohydrate specific), in both groups (Common), and only in ethanol group (Ethanol specific) for C/EBP-β and C/EBP-α
| Ethanol Specific | Common | Carbohydrate Specific | ||||
|---|---|---|---|---|---|---|
| Time, h | 0 | 6 | 0 | 6 | 0 | 6 |
| C/EBP-α | 592 | 522 | 634 | 519 | 2,173 | 2,092 |
| C/EBP-β | 4,251 | 2,035 | 97 | 2,285 | 23 | 3,024 |
COMPACT Analysis Revealed Ethanol-mediated Alternative Binding Dynamics for C/EBP-α and C/EBP-β
We used our novel COMPACT analysis to identify altered C/EBP-β and C/EBP-α binding dynamics caused by chronic ethanol adaptation (Fig. 1, A and B). A COMPACT matrix approach provides an alternative to the conventional treatment vs. control analysis. Here, the genome-wide binding effects of two conditions, termed “comparative pair,” are evaluated at multiple levels of another factor, termed “pattern set” (time points). The complete set of comparative pairs representing the intersection between Treatment vs. Control yields a COMPACT matrix (36, 37) (http://compact.jefferson.edu). The elements of the COMPACT matrix are based on pair-wise gene counts of the corresponding patterns, i.e., for the comparative pair Treatment vs. Control, the element at the ith row and jth column of the matrix contains the number of genes that show an ith binding pattern in Control and jth binding pattern in Treatment. In addition to providing a systems-level distribution of gene sets among patterns, COMPACT provides a layered approach that aids in the systematic exploration of the binding patterns that are altered in specific ways due to treatments. We visualized the changes to binding dynamics using a chord diagram representation (Fig. 1, C and D). We arranged similar responses in the diet groups symmetrically on both sides of the circle, with arcs representing temporal binding patterns. The segmented arcs of a circle correspond to the individual binding patterns (i.e., rows and columns of COMPACT matrix), with the width of the chord proportional to the number of genes exhibiting the corresponding binding pattern. Binding patterns are arranged in an order that separates baseline binding from post-PHx binding.
For C/EBP-β, of all the loci that showed binding in either dietary group, a set of 1,811 genes showed binding only in the ethanol group (i.e., no-binding in control) (total of first row in the COMPACT matrix shown in Fig. 1A; yellow arc on the control side in Fig. 1C), compared with 3,117 genes with no-binding in the ethanol group (total of first column in the COMPACT matrix shown in Fig. 1A; yellow arc on the ethanol side in Fig. 1C). There were significant differences in the number of C/EBP-β binding loci at baseline between the dietary groups (2,119 genes in the Carbohydrate group vs. 26 in the Ethanol group; refer to second row and column, respectively, in the COMPACT matrix shown in Fig. 1A). In the case of C/EBP-α, the no-binding pattern showed a reverse trend. The set of genes that showed lack of binding was larger in control diet (Fig. 1D, yellow arc on the control side) compared with that of the ethanol diet group (Fig. 1D, yellow arc on the ethanol side). Baseline binding occurred in both diet groups (Fig. 1D, green arcs) with the ethanol group showing a higher number of putative targets in the adapted state. Within the baseline-bound genes in the ethanol group, the largest fraction was from the set of genes that were not bound in the control group (Fig. 1D, yellow ribbon connected to the green arc on the ethanol side). Similar analysis revealed a relatively higher fraction of genes bound only at 6 h post-PHx (Fig. 1D, red arc on the ethanol side) for both of the transcription factors.
The COMPACT analysis highlighted dominant C/EBP-β binding patterns (Fig. 1, A and B). Baseline patterns revealed that the overall genes bound in the ethanol group at baseline alone (26) were significantly lower than that of the control group (2,119). Inspection of the COMPACT matrix elements showed that, at baseline alone, the only dominant group was bound dynamically in the control group but showed no binding in the ethanol group (1,342 in Fig. 1A, large green ribbons connecting the green control arc with the yellow ethanol arc).
C/EBP-β binding showed a high number of carbohydrate-specific targets at baseline and post-PHx (3,117 total bound genes, first column in COMPACT Fig. 1A). One of the dominant patterns includes a set of genes that were not bound in the ethanol group but were bound only post-PHx in the control group (1,235 genes in Fig. 1A; large red and green ribbons connecting the red and green control arcs, respectively, with the yellow ethanol arc). However, our COMPACT analysis revealed altered binding dynamics post-PHx in the ethanol group compared with baseline binding in the ethanol group, as indicated by the set of genes that were not bound in the control group but were bound only post-PHx in the ethanol group (1,794 genes in Fig. 1A; large yellow ribbon connecting yellow control arc with the red ethanol arc in Fig. 1C). Another dominant group included genes persistently bound in the control group, but bound only post-PHx in the ethanol group (1,609 genes in Fig. 1A; large blue ribbon connecting blue control arc and red ethanol arc in Fig. 1C). Carbohydrate control rats showed a slight increase in C/EBP-β binding post-PHx (172 additional bound genes), while ethanol-adapted rats showed an accentuated increase in C/EBP-β binding peaks post-PHx (5,187 additional genes). The largest pattern (1,794 genes) was ethanol diet-specific binding at 6 h post-PHx (Fig. 1C). These results indicate that C/EBP-β may regulate novel transcriptional functions following the combined stresses of ethanol-adaptation and PHx.
Such a dramatic alteration to dynamic binding post-PHx due to ethanol adaptation, however, was not evident in the case of C/EBP-α binding. At baseline, the major contribution was from the ethanol group (1,271 genes) with no binding in the control group. This suggests that chronic ethanol intake causes an overall increase in C/EBP-α binding. Post-PHx, the striking feature highlighted by the chord diagram is the overall higher number (3,056 genes) of ethanol-specific targets (i.e., no binding in the carbohydrate control group) (Fig. 1B). However, in the case of C/EBP-α binding, the alteration in number of bound genes from the baseline state to the post-PHx state was not as striking as in the case of C/EBP-β binding. We found that the control group showed a slight decrease in binding from baseline to 6 h post-PHx (893 – 708 = 185 fewer genes; rows 2 and 3 in Fig. 1, B and D). Such a reduction in the number of bound loci is consistent with previous results showing a decrease in the level of C/EBP-α DNA binding activity post-PHx (12, 44). Ethanol-adapted liver showed a similar decrease in C/EBP-α binding post-PHx (1,680 – 1,484 = 196 fewer genes; columns 2 and 3 in Fig. 1, B and D), but the overall number of genes bound by C/EBP-α following ethanol adaptation was more than double the number of genes bound by C/EBP-α in controls (1,680 + 1,484 + 1,127 = 4,291 vs. 893 + 708 + 333 = 1,934 total genes bound). A large majority of these genes showed ethanol-specific binding (3,056 genes; top row in COMPACT, Fig. 1B), indicating that ethanol adaptation may cause C/EBP-α to regulate novel functions in the homeostatic state, as well as in response to an acute challenge such as PHx. The chord diagram visualization further allows for identification and isolation of dominant patterns of transcription factor binding that may otherwise be less apparent in a COMPACT matrix. For example, we can investigate ethanol-specific, common, and carbohydrate-specific genes bound by C/EBP-β (Fig. 1E) and C/EBP-α (Fig. 1F) at baseline, at 6 h post-PHx, and with persistent binding.
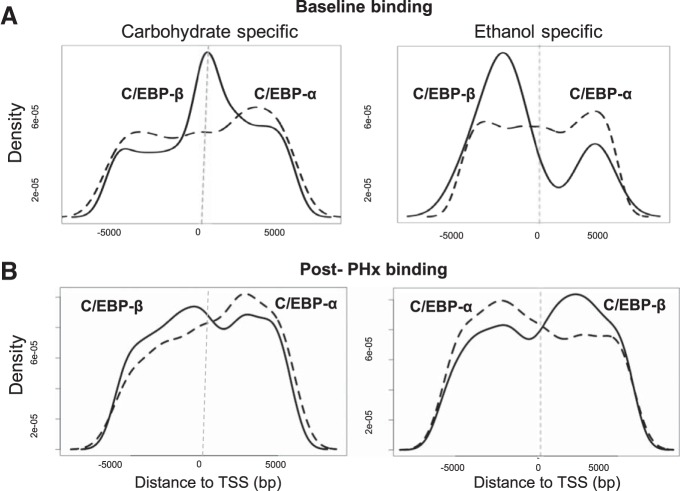
Chronic Ethanol Consumption Induced a Shift in the Preferential Proximal Promoter Binding Loci of C/EBP-α and C/EBP-β
We investigated whether chronic alcohol consumption changed how C/EBP-α and C/EBP-β bind to their target genes by calculating the distribution of binding peaks relative to the transcription start site (TSS) for C/EBP-α and C/EBP-β. The distance to TSS was calculated as the peak start position minus the TSS position. We first examined the distribution of the binding peaks with respect to TSS in the baseline state for control as well as ethanol diet groups. Adaptation to chronic ethanol intake led to a shift in C/EBP-β binding from upstream to further downstream for several targets, while the C/EBP-α binding distribution was unaffected relative to TSS (Fig. 2A). However, post PHx, the ethanol group showed a switch in the peak of binding locus distribution for both C/EBP-α and C/EBP-β (Fig. 2B). In the control group, C/EBP-α binding showed a peak at ~2700 bp downstream of the TSS, while C/EBP-β binding showed a peak at ~700 bp upstream of the TSS. The ethanol group showed nearly opposite binding locus peaks post PHx, with the C/EBP-α binding peak occurring at ~2,100 bp upstream of TSS and the C/EBP-β binding peak at ~2,400 bp downstream of TSS (Fig. 2B). This ethanol-mediated reversal of transcription factor peak binding distribution suggests global changes in C/EBP regulation of transcription following PHx. Although this ethanol-mediated shift in preferential proximal promoter binding occurred for both C/EBP-α and C/EBP-β, the effects of such a shift on transcriptional regulation of target genes are as yet unclear.
Fig. 2.
Effects of adaptation to chronic ethanol intake on C/EBP binding. A: carbohydrate-specific genes show peak C/EBP-β binding around transcription start site (TSS), while C/EBP-α binding peaks downstream of the TSS. Chronic ethanol adaptation shifted C/EBP-β binding to further upstream, while C/EBP-α binding is unaffected. B: carbohydrate-specific genes show peak C/EBP-β binding upstream of the TSS, while C/EBP-α binding peaks downstream of the TSS. Chronic ethanol adaptation reversed this pattern of binding in ethanol-specific genes.
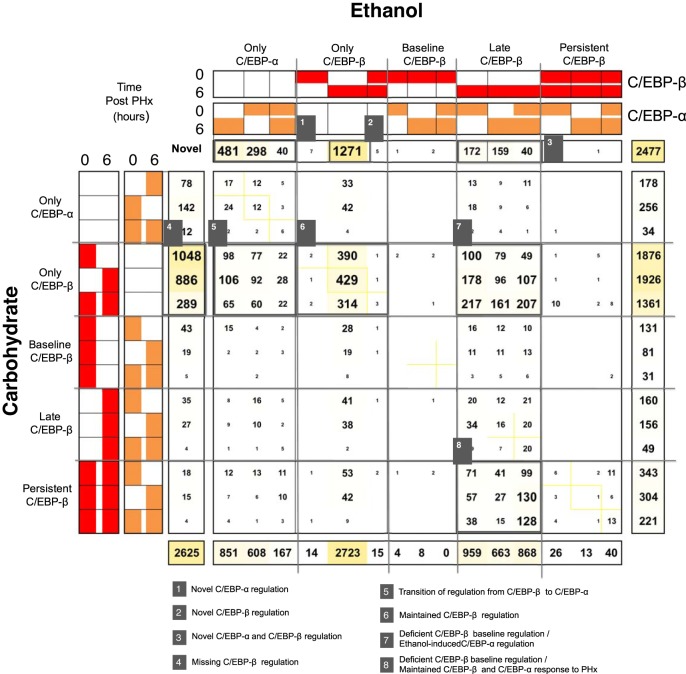
Functional Analysis Revealed Ethanol-mediated Adaptation of the C/EBP Transcriptional Switch
It has been reported that C/EBP-α and C/EBP-β function as a transcriptional regulatory switch rather than as independent and uncoordinated transcription factors (28). We therefore extended our COMPACT analysis to compare dynamic patterns of combinatorial C/EBP binding (Fig. 3). This approached allowed for identification of several sets of genes (marked in Fig. 3) with altered C/EBP binding due to chronic ethanol adaptation: (set 1) Ethanol-specific C/EBP-α binding, (set 2) Ethanol-specific C/EBP-β binding, (set 3) Ethanol-specific C/EBP-α and C/EBP-β binding, (set 4) Carbohydrate-specific C/EBP-β binding, (set 5) Common binding that switches from C/EBP-β in controls to C/EBP-α in the ethanol group, (set 6) Common binding that maintains C/EBP-β binding, (set 7) Common binding that switches from C/EBP-β in controls to both C/EBP-β and C/EBP-α binding in the ethanol group, and (set 8) Common binding that is regulated by both C/EBP-β and C/EBP-α but has deficient C/EBP-β biding at baseline in ethanol-fed rats.
Fig. 3.
COMPACT analysis of C/EBP binding in ethanol-fed rats and controls. The intersection between the Ethanol vs. Carbohydrate pattern count distributions exhaustively considers all possible comparative patterns for the combinations of C/EBP-β and C/EBP-α binding, yielding a COMPACT matrix. The elements of the COMPACT matrix are based on pair-wise gene counts of the corresponding patterns, i.e., for the comparative pair, the element at the ith row and jth column of the matrix contains the number of genes that show an ith binding pattern in Carbohydrate and jth binding pattern in Ethanol diet. The diagonal of the matrix represents those genes showing a common response and the off-diagonal elements represent the genes showing an altered temporal binding response between the 2 comparative conditions. We identified 8 major binding patterns of C/EBP binding.
Sets 1, 2, and 3 contain genes that showed C/EBP binding only after chronic ethanol adaptation. Set 4 contains genes that have deficient C/EBP-β binding following chronic ethanol adaptation. Sets 6 and 8 contain genes that have similar binding across treatments, but set 8 has deficient baseline C/EBP-β binding after chronic ethanol adaptation. Sets 5 and 7 contain genes with C/EBP-β binding in control but with C/EBP-α regulation that occurs as a result of ethanol adaptation.
A detailed pathway analysis of these target sets revealed that they are involved in functions that are critical to the liver regeneration process, and are summarized as below. The complete list of pathway analysis results is included in Table 2.
Table 2.
Genes and processes involved in each of the 8 major C/EBP binding patterns from Fig. 3
| Group | Genes, n | Description | Select Genes | Processes/Functions |
|---|---|---|---|---|
| 1 | 819 | Novel C/EBP-α regulation | Pdcd4, Sv2c, Mmp9, Clic4, Col1a1, Irak4, Raf1, Tnnt2, Mapk6, Chd2, Hpgd, Tlbp2, Tlbp4, Pdgfrb | Tgfb signaling pathway, regulation of cell growth, cell adhesion, kinase regulator activity, cell fraction, membrane, phosphorylation |
| 2 | 1,271 | Novel C/EBP-β regulation | Cebpg, Cebpe, Egf, Car2, Adamts1, Ak3l1, Casp12, Ccr7, Chd1, Col3a1, Cyp39a1, Edn1, Igfbp1, Lrat, Lum, Nt5e, Sparc, Tlr3, Mmp12, Traf6 | Regulation of protein kinase cascade, fat cell differentiation, response to wounding, regulation of homeostatic process, positive regulation of apoptosis, cell fraction, regulation of biosynthetic process |
| 3 | 371 | Novel C/EBP-α and C/EBP-β regulation | Tgfb3, Mapk1, Fos, Egr3, Il7, Tnsf11 | Cell proliferation, cell-cell signaling, regulation of kinase activity, |
| 4 | 2,223 | Missing C/EBP-β regulation | Nfkb2, Nod2, Rbpj, Vcam1, Sox1, Sox2, Vegfc, Anxa5, Bcl10, Acvr1b, Aimp1, Alox12, Anxa1, Ccne1, Ch25h, E2f1, E2f3, Itga1, Sulf2, Usp14, C6, C9, Faslg | Apoptosis, cellular protein catabolic process, fatty acid metabolic process, glycolysis |
| 5 | 570 | Transition of regulation from C/EBP-β to C/EBP-α | Cebpa, Tgfa, Tm4 sf1, Gcnt2, Nf1, Pdgfb, Jak2, Smad4 | Cell motion, cell migration, regulation of cellular biosynthetic process, cell fraction, phosphoprotein |
| 6 | 1,133 | Maintained C/EBP-β regulation | Jun, Ctgf, Hif1a, Atr, Prkcb | Phosphatase activity, homeostatic process, cell fraction, cell projection, cell adhesion, ATP metabolic process, ion binding |
| 7 | 1,194 | Deficient C/EBP-β baseline regulation/Ethanol-induced C/EBP-α regulation | Tgfb2, Npy, Ets2, Bach1, Npas2, Ntf3, Avpr1a, Bcl6, Ccrn4l, Cxcl12, Cyp2681, Ifng, Il15, App, Cd24 | Regulation of apoptosis, cell adhesion, cell-cell signaling, cAMP-mediated signaling, protein kinase activity, cell growth, membrane, ion binding |
| 8 | 606 | Deficient C/EBP-β baseline regulation/Maintained C/EBP-β and C/EBP-α response to PHx | Tgif1, Smad3, Ccnd2, Fgfr2, Lama1, Rxra, Tgfbr2 | Cell morphogenesis, regulation of cell proliferation, transcription repressor activity |
Set 1: Loci with ethanol-specific C/EBP-α binding (819 genes).
The genes in this pattern are putative C/EBP-α-regulated, ethanol-specific genes. Specific pathways such as the TGF-β signaling pathway (e.g., Hpgd, Pdgfrb, Ltbp2, Ltbp4, Usp96) and EGF signaling pathway are regulated by some of these C/EBP-α bound target genes. This particular pathway is of interest because TGF-β has been shown to negatively impact liver regeneration through suppression of the hepatocyte cell cycle and through activation of hepatic stellate cells (2). Additionally, C/EBP-α and TGF-β are known to suppress each other, indicating that ethanol-specific regulation of the TGF-β pathway by C/EBP-α may suppress TGF-β signaling or hepatocyte response to TGF-β signaling (48, 56). These results suggest that chronic ethanol intake may modulate C/EBP-α regulation of TGF-β signaling during the priming phase of liver regeneration. Pathway analysis revealed that, in addition to the above key pathways, these genes were also associated with functions such as cell growth, homeostasis, regulation of kinase activity, cell membrane potential (for example Clci4), and cell adhesion.
Set 2: Loci with ethanol-specific C/EBP-β binding (1,271 genes).
The genes in this pattern are putative C/EBP-β-regulated, ethanol-specific genes. C/EBP-β gene expression is known to be activated by ethanol (6). Previous studies have showed that collagen production in hepatic stellate cells due to ethanol intake is mediated by C/EBP-β DNA binding (65). We found several genes related to collagen production regulated specifically in ethanol-adapted rats (Adamts1, Col3a1, Edn1, Lrat, Sparc, Mmp12). These promoters were bound and may be regulated by C/EBP-β after ethanol adaptation, thereby contributing to the inhibitory effects of ethanol adaptation to liver regeneration post-PHx.
Pathway analysis revealed that genes associated with these dynamic patterns regulate functions associated with facilitation of regeneration, such as response to wounding and regulation of protein kinase cascade, chromosome organization (for example, Chd1), as well as functions associated with inhibiting regeneration, such as regulation of homeostatic process and positive regulation of apoptosis. These results suggest that chronic adaptation to ethanol may cause C/EBP-β regulation of both pro-regenerative and anti-regenerative functions post-PHx.
Set 3: Loci with Ethanol-specific C/EBP-β and C/EBP-α binding (371 genes).
These loci were bound to C/EBP-α in several dynamic patterns but were consistently bound to C/EBP-β in response to PHx. These loci were also bound by the C/EBPs only in ethanol-adapted rats. We were interested to see that the genes in these patterns contained both genes thought to enhance regeneration (for example, Fos, Mapk1, Il7) and genes thought to inhibit regeneration (for example Tgfb3), suggesting a dual role of chronic ethanol adaptation in promoting and inhibiting regeneration. In addition, we found putative target genes in these dynamic binding patterns to be related to functions including cell proliferation and regulation of kinase activity.
Set 4: Loci with carbohydrate-specific C/EBP-β binding (2,223 genes).
These dynamic regulatory patterns contained the largest number of genes of all the groups analyzed. Genes in these dynamic patterns were regulated by C/EBP-β in response to PHx in only the carbohydrate-diet control rats. We were interested to find several genes identified as important to growth, immune and metabolic response (for example, Nfkb2, Vcam1, E2f3, Sox2, Nod2, Vegfc, Bcl10) and apoptosis (for example, Rbpj, E2f1, Rarg, Traf2, Timp3) as part of this group. These genes being regulated post-PHx uniquely in the carbohydrate-specific set indicates that chronic ethanol adaptation may cause deficient regulation of these genes and contribute to inhibiting the liver’s ability to regenerate post-PHx.
Set 5: Loci showing transition of binding from C/EBP-β to C/EBP-α (570 genes).
Loci in this set were bound by C/EBP-β in controls, but their putative regulation switched to C/EBP-α binding activity following chronic ethanol adaptation. Of particular interest was that C/EBP-α itself belonged to this set, indicating that following ethanol adaptation and PHx, C/EBP-α may be acting through a positive feedback to maintain high expression and activity. Such an amplified C/EBP-α activity may increase the C/EBP-α to C/EBP-β ratio post PHx, which has been shown to inhibit liver regeneration (18). A functional analysis of the genes in this set revealed that these genes were related to processes such as cell motion, cell migration, and regulation of cellular biosynthetic process. We found a small set of genes (including Enpp1, Insig1, and Wwtr1) that negatively regulate fat cell differentiation in this set.
Set 6: Loci with maintained C/EBP-β binding 6 (1,113 genes).
Loci in this group were bound by C/EBP-β in both control and ethanol-adapted rats. We therefore expected genes in this set to relate to liver functions outside the context of regeneration. A functional analysis revealed that these genes were associated with functions such as homeostatic processes (for example, Cdk6, Ins2, Dbh, Prkcb, Hif1a, Pgm1), cell adhesion (Cd9, Ctgf, Ptprc) and ATP metabolic process (Gata4).
Set 7: Loci with deficient C/EBP-β baseline binding or ethanol-specific C/EBP-α binding (1,194 genes).
Loci in this set were bound only by C/EBP-β in controls but were bound by both C/EBP-β and C/EBP-α in ethanol-adapted rats. The additional C/EBP-α binding may indicate an augmented inhibitory regulation of these genes following ethanol adaptation. Several proinflammatory genes were contained in this set (including Cxcl12, Cd24, Ifng, Bcl6, Il15) as well as genes implicated in hepatic stellate cell activation that may be inhibitory to regeneration (for example Tgfb2, Npy). A functional analysis of genes contained in this set revealed that they are related to functions involved in liver regeneration, including regulation of apoptosis, cAMP-mediated signaling, protein kinase activity, and cell growth. The altered regulation of cell growth may indicate that ethanol adaptation causes and imbalance in the liver proliferation/growth response to PHx, which has been predicted to be important for overall mass recovery (8).
Set 8 Loci with deficient C/EBP-β baseline binding or maintained C/EBP-β and C/EBP-α binding in response to PHx (606 genes).
Loci in this set were bound by both C/EBP-α and C/EBP-β in both control and ethanol-adapted rats. In the control rats, however, these loci were bound at baseline and post-PHx by C/EBP-β, while in ethanol-adapted rats, these loci were bound by C/EBP-β only post-PHx. Genes included in this group were related to TGF-β signaling components, such as the TGF-β receptor Tgfbr2 and Smad3. Additionally, we found that the cell cycle gene Ccnd2 was associated with this group. The altered dynamic regulation of these genes by C/EBP-β in ethanol adapted rats may lead to deficiencies in TGF-β signaling or in cell cycle progression, which could impact regeneration dynamics. A functional analysis revealed that the corresponding genes were related to functions such as regulation of cell proliferation and transcriptional repressor activity.
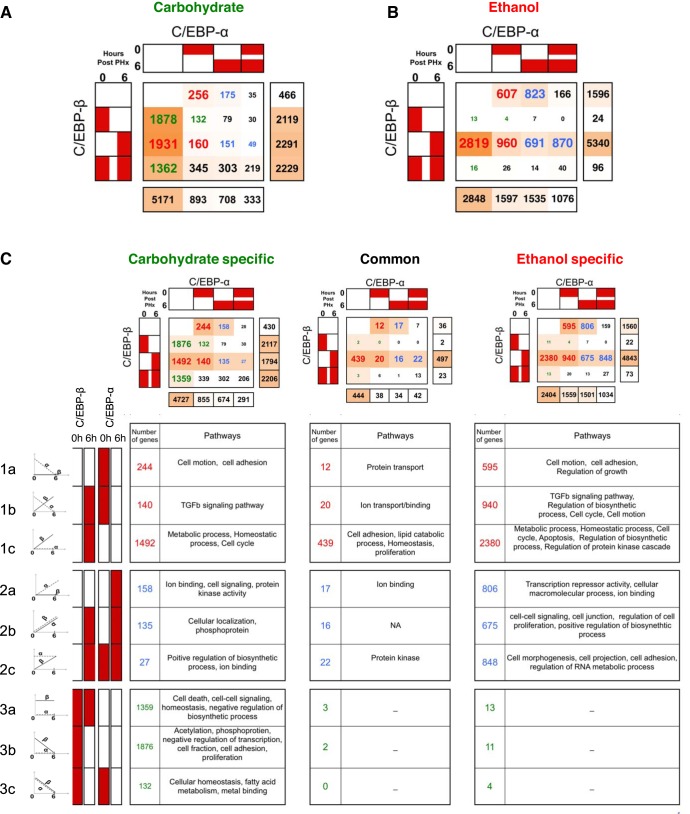
Diet-Specific COMPACT Analysis Revealed Functional Effects of Chronic Ethanol-Adaptation on C/EBP Binding during the Priming Phase of Liver Regeneration
The COMPACT analysis so far focused on how groups of genes were differentially regulated due to ethanol intake. We further investigated the dynamics of C/EBP regulation of target genes using a complimentary COMPACT approach focusing on the regulatory switches rather than the regulated genes. We first constructed COMPACT matrices showing C/EBP-β vs. C/EBP-α binding for control and ethanol diets independently (Fig. 4, A and B). We divided these matrices into three different COMPACT matrices showing carbohydrate-specific dynamics, common dynamics, and ethanol-specific dynamics (Fig. 4C). Thus, for each matrix element, the sum of the same elements from the carbohydrate (Fig. 4A) and ethanol (Fig. 4B) matrices equals the sum of that same element from the carbohydrate-specific, common, and ethanol-specific matrices (Fig. 4C). This approach resulted in smaller COMPACT matrices, allowing for easier pattern recognition and visualization of results to focus our attention on how ethanol consumption alters the dynamic regulation of functions by the CEBP switch. We were interested in investigating the major functions regulated by the CEBP switch in terms of ethanol-specific, common, and carbohydrate-specific dynamics. We therefore focused our investigation on the functions regulated by three major patterns of C/EBP binding containing a large number of target genes: decreasing or absent C/EBP-α binding, increasing or sustained C/EBP-α binding, and baseline C/EBP-β binding. Each of these major patterns were further divided into specific patterns of C/EBP binding.
Fig. 4.
COMPACT analysis of the C/EBP binding switch. A: gene groups corresponding to various combinations of the C/EBP-α and C/EBP-β binding switch in control rats and ethanol-adapted rats. B: gene groups corresponding to the C/EBP-α and C/EBP-β binding switch in carbohydrate-specific, common, and ethanol-specific dynamic regulation. C: statistically significant pathways corresponding to the dynamic C/EBP binding switch in a carbohydrate-specific, common, and ethanol-specific manner.
Decreasing/increasing or absent C/EBP-α and C/EBP-β binding.
The ethanol-specific group showed higher C/EBP binding in all three dynamic patterns (decreasing or absent C/EBP-α binding, increasing or sustained C/EBP-α binding, and baseline C/EBP-β binding) than either the common or carbohydrate-specific groups. Although these three dynamic patterns also show a high amount of C/EBP binding in the carbohydrate-specific group, there were few genes that were bound in these patterns in both control and following ethanol adaptation (common group). This indicates that for these dynamic patterns, ethanol adaptation caused a shift from a healthy response to a nearly mutually exclusive maladaptive response. We therefore investigated the pathways regulated in common, ethanol-specific, and carbohydrate-specific response to PHx in each of the dynamic patterns using the Database for Annotation, Visualization and Integrated Discovery (DAVID) online resource (25).
dynamic pattern 1a (00-10): c/ebp-α unbinding in response to phx.
We found that ethanol adaptation caused a larger C/EBP-α unbinding response than controls with no C/EBP-β regulation (595 vs. 244 genes). This increased C/EBP-α unbinding response was coupled with a baseline increase in C/EBP-α binding due to chronic ethanol adaptation, indicating that C/EBP-α binding was deregulated following ethanol-adaptation. But the additional intervention of PHx may act to suppress C/EBP-α binding to these targets unbound in control rats. Our pathway analysis revealed that in control rats, the genes bound in this pattern regulate cell motion and cell adhesion (Fig. 4C). In ethanol-adapted rats, in contrast, the bound genes regulated cell motion, cell adhesion, and regulation of growth. These findings indicate that, at baseline, C/EBP-α binding may act as a novel negative regulator of cell growth, a function not targeted by C/EBP-α in control-fed rats.
dynamic pattern 1b (01-10): c/ebp-α unbinding coupled with cebp-β binding in response to phx.
In the carbohydrate-specific set of genes, we found relatively few genes within this pattern. In the ethanol-specific genes, by contrast, we found a relatively high number of genes within this pattern. Based on previous studies suggesting that C/EBP-α binding was inhibitory to liver regeneration and C/EBP-β binding enhanced regeneration, we expected a large number of genes to fall into this pattern in control-fed rats (18). The finding that ethanol-adapted rats showed a higher number of genes with this behavior was therefore unexpected. However, this subset of genes is only a part of a larger set of genes that showed alteration in C/EBP-α binding. Examining the overall increase in C/EBP-α specific binding post PHx (control, 674; ethanol, 1,501) indicated that there is an equal proportion of genes that are bound as novel targets in the ethanol diet. This suggests that the increased number of genes displaying this dynamic binding pattern in ethanol-adapted rats may reflect a compensatory mechanism, designed to alleviate other ethanol-induced deficiencies in C/EBP binding. Alternatively, this binding may activate a set of genes ultimately inhibitory to regeneration that are not normally activated early post-PHx. Our pathway analysis revealed that the genes bound in control rats in this pattern regulated ion transport and the TGF-β signaling pathway (Common, Carbohydrate specific). In ethanol-adapted rats, the genes in this pattern regulated the TGF-β signaling pathway and the cell cycle, as well as other functions (Fig. 4C). Many of the genes bound in the ethanol-specific pattern have been implicated in hepatic stellate cell activation, fibrosis developme nt, and inhibition of cell proliferation. This suggests that the dynamic binding of C/EBP may play an important role in regulating hepatic stellate cell activation, which may be enhanced following chronic ethanol adaptation.
dynamic pattern 1c (01-00): c/ebp-β binding in response to phx.
In all sets of genes (Ethanol specific, Common, and Carbohydrate specific), this pattern contained a relatively high number of genes, with the ethanol-specific set containing the highest number (Fig. 4A). This result is consistent with previous studies showing that C/EBP-β binding is enhanced following ethanol intake (6, 53). However, the functional understanding of the increase in binding in the ethanol-specific set is unclear, because of the lack of regenerative response seen in these animals (34). Our pathway analysis of these genes revealed that both the ethanol-specific pattern and the carbohydrate-specific pattern regulated genes were associated with the cell cycle. The ethanol-specific pattern also regulated apoptosis, which was missing in the carbohydrate-specific and common binding patterns. This result indicates that, while both control and ethanol-adapted rats displayed transcriptional regulation of the cell cycle, chronic ethanol adaptation may increase apoptosis propensity post-PHx, negatively affecting overall liver mass recovery and tissue function.
Increased or sustained C/EBP-α binding.
The ethanol-specific set showed higher binding in these patterns than the carbohydrate-specific set; the common set again contained few genes in these patterns, indicating that chronic ethanol adaptation alters dynamic regulation by C/EBPs. The increased number of genes in these patterns in the ethanol-specific set may also point to an imbalance in the ratio of C/EBP-α to C/EBP-β binding caused by chronic ethanol adaptation.
dynamic pattern 2a (00-01): c/ebp-α binding in response to phx.
Previous studies have shown that C/EBP-α binding in response to PHx impairs liver regeneration (12, 44). We found a large increase in C/EBP-α specific binding in response to PHx, in the ethanol-specific group (806 vs. 158) (Fig. 4B). This may contribute to ethanol-induced impairment of liver regeneration. Our functional analysis showed that the ethanol-specific set contained C/EBP-α bound to genes regulating transcription repressor activity while the carbohydrate-specific set contained C/EBP-α bound to genes regulating protein kinase activity, suggesting a mechanism of ethanol-impaired regeneration through deficient protein kinase activity coupled with transcriptional repression.
dynamic pattern 2b (01-01): combined cebp binding in response to phx.
We found that chronic alcohol consumption caused an increase in genes associated with this pattern (675 Ethanol specific, 135 Carbohydrate specific) (Fig. 4B). The ethanol-specific set contained genes related to cell-cell signaling and regulation of cell proliferation. Specific genes in this pattern indicate hepatic stellate cell-specific C/EBP binding response to PHx (Npy, Smad2) (Supplemental Table S1) and may be related to hepatic stellate cell activation novel to chronic ethanol adaptation.1 Notably missing from the Ethanol-specific set of genes in this pattern was C/EBP regulation of Hgf, which was present in the carbohydrate-specific set (Supplemental Table S1). Previous studies also indicated C/EBP upregulation in stellate cells leading to expression of Hgf (11). Hence, a C/EBP-mediated Hgf deficiency may contribute to ethanol-impaired regeneration.
dynamic pattern 2c (01-11): sustained c/ebp-α, c/ebp-β binding response to phx.
The ethanol-specific set of genes in this pattern contained a much higher number than the carbohydrate-specific set (848 vs. 27) (Fig. 4B), indicating that chronic ethanol adaptation may result in a higher sustained C/EBP-α binding throughout the genome. Carbohydrate-specific genes in this pattern were associated with protein kinases, indicating that ethanol-fed animals may have deficient regulation of these kinases, contributing to an inability to respond to growth factor signaling post-PHx.
Baseline C/EBP-β binding.
As opposed to the other patterns discussed, the carbohydrate-specific set contained 1–2 order of magnitude higher numbers of genes in these patterns than either the common set or the ethanol-specific set. This result indicates that functions governed by these genes may be deficient following PHx in ethanol-adapted rat livers.
dynamic pattern 3a (11-00): sustained c/ebp-β binding, no c/ebp-α binding
The ethanol-specific set of genes appears to be deficient in sustained C/EBP-β regulation of cell death, signaling, and homeostasis functions (Fig. 4C). Additionally, several genes important to the early proinflammatory response of the liver to PHx appeared in this group, including Jun, Ifng, and Tgfb2 (Supplemental Table S1). Taken together, these results suggest that ethanol-adapted rats have deficient cytokine regulation post-PHx that may lead to altered NF-κB activity or binding and may influence the progrowth/proapoptotic role of NF-κB (32, 46).
dynamic pattern 3b (10-00): No c/ebp-α binding, c/ebp-β unbinding in response to phx.
The C/EBP-β unbinding response to PHx seen in the carbohydrate-specific set of genes may lead to a decreased activity of several functions regulated by genes in this pattern, including acetylation, phosphorylation, and negative regulation of transcription (Fig. 4C). Taken together, the functions associated with this C/EBP-β unbinding pattern may indicate that, in response to PHx, homeostatic C/EBP-β binding could be relocated from maintaining low transcription and promoting DNA acetylation and protein phosphorylation to initiating a transcriptional program supporting hepatocyte entry into the cell cycle.
dynamic pattern 3c (10-10): c/ebp-α and c/ebp-β unbinding in response to phx.
Similar to the above pattern, C/EBP binding to genes in this pattern (Fig. 4C) may be important for homeostatic liver function but unnecessary during the priming phase of regeneration. Functions associated with genes in this pattern in the carbohydrate-specific set include cellular homeostasis and fatty acid metabolism.
Potential Transcriptional Co-regulation with C/EBP-α and C/EBP-β Post-PHx
Putative mechanisms for transcriptional co-regulation was determined by the organization of regulatory elements distributed along the genome and their colocation with the C/EBP-α and C/EBP-β binding loci. These two members of the C/EBP family showed high levels of nucleotide homology with consensus sequences (ATTGCGCAAC) within the basic region and leucine zipper domains (20). We performed a search for enriched de novo motifs on the genomic locations corresponding to the dynamic patterns of genome-wide C/EBP promoter binding in control and ethanol data for three dynamic patterns of interest (C/EBP-α unbinding in response to PHx, C/EBP-β binding in response to PHx, and a switch from C/EBP-α to C/EBP-β regulation in response to PHx) using the motif discovery and analysis tool DME (54). We also employed PAINT (58), which acts as a convenient interface to the TRANSFAC Pro transcription factor binding site database and the associated MATCH pattern-matching tool (40). Total coverage was calculated for uniquely enriched sequences from both DME and PAINT. We visualized the results using the online tool STAMP (39). Overall, our results identified SMAD3 and AP-1 as the most common transcription factors that appear to regulate genes in coordination with C/EBP-α and C/EBP-β (Fig. 5). We examined each group included in our analysis in detail below to investigate co-regulation beyond these two transcription factors.
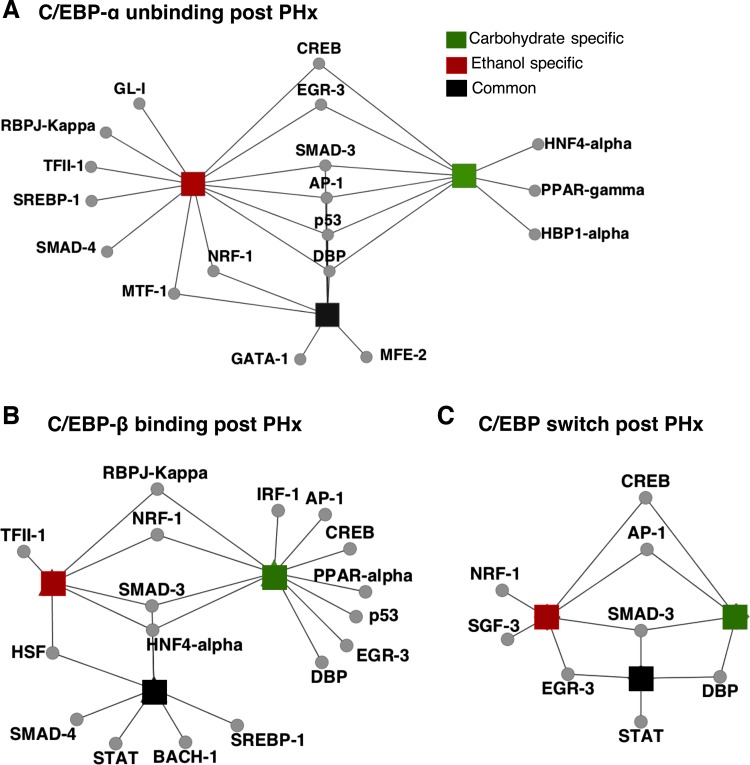
Fig. 5.
Co-regulatory factors potentially acting in concert with C/EBPs in distinct C/EBP binding patterns. A: C/EBP-α unbinding, B: C/EBP-β binding, C: C/EBP binding switch.
C/EBP-α unbinding in response to PHx.
In this group, the genomic loci were enriched for motifs for AP-1, SMAD3, p53, EGR-3, and CREB in all three groups: control-specific, common, and ethanol-specific (Supplemental Table S2). These transcription factors are associated commonly with functions such as metabolism, homeostasis, and development. We also found enriched in all three groups DBP, a liver-enriched transcription factor that binds to similar consensus sequences as C/EBPs (50). We found novel cobinding sites for SMAD4, RBPJ-K, and SREBP-1 in the ethanol-specific group only (Fig. 5A). This result suggests that one mechanism by which chronic ethanol abuse may disrupt the balance of lipid metabolism is through increased activity of the transcription factor SREBP-1 (66), the expression of which is directly regulated by C/EBPs in the liver (45). SMAD3 and SMAD4, which are both enriched in the ethanol-specific group, have been shown to repress transcription by inhibiting the transactivation function of C/EBP and activating TGF-β pathways (7). Although this result was shown in the context of adipogenesis, a similar mechanism may be active in the livers of ethanol-adapted animals contributing to suppressed regeneration.
HNF-4a, PPAR-γ/RXRa, and IRF-1 were found enriched only in the carbohydrate-specific group. HNF-4a is a well-known target of C/EBPs, and coexpression of HNF-4a with C/EBP-α has been observed in human HCC cell lines (27, 49). The lack of HNF-4 co-regulation of target genes with C/EBPs in ethanol-fed animals may indicate a deficiency that inhibits C/EBP target gene transcription compared with controls. Similar to HNF-4a, genome-wide binding of PPAR-γ on mouse adipocytes using ChIP-chip showed that both PPAR-γ and C/EBP-α bound genes induced adipogenesis (7). Again, the deficient co-regulation in the ethanol-fed animals indicates a lack of a potentially important cofactor for target transcription.
In the common group, we found the known motif for the metal response factor MTF-1 (28). We also found several novel motifs for NRF-1 and MEF-2 (Supplemental Table S2). NRF-1 has been implicated previously in organ regeneration and brain ischemia, indicating that the promoters co-regulated by NRF-1 and C/EBPs may contribute to the liver response to hypoxia during regeneration.
C/EBP-β binding in response to PHx.
We found that SMAD3 and HNF4a co-regulated target genes with C/EBPs in all three groups (Fig. 5B). The common group was enriched for SMAD4, STAT, BACH1, and SREBP-1 (Supplemental Table S2). Both the ethanol group and the control group were enriched for NRF-1 and RBPJ-K. Some known C/EBP-β motifs such as AP-1, CREB, PPAR-A/RXR-a, p53, and IRF-1 were found to be carbohydrate specific. These transcription factors were associated with functions including transcription factor binding, transcription, growth factor response, and fatty acid oxidation. The deficient co-regulation of these transcription factors with C/EBPs in the ethanol-adapted state points to another potential defect contributing to ethanol-impaired regeneration.
Switch from C/EBP-α to C/EBP-β regulation in response to PHx.
The transcription factor co-regulating target genes with C/EBPs in this pattern may contribute to governing C/EBP switch-like behavior following resection. We found SMAD-3 motif in all three groups (Supplemental Table S2), suggesting that SMAD-3 could be an important regulator of the C/EBP switch. We found the AP-1 motif to be an ethanol and control-specific motif, implicating it as well in control of the C/EBP switch. Additionally, we found the STAT3 motif in the common set (Supplemental Table S2), which suggests STAT3 could also play a role governing the C/EBP switch. We also found several novel motifs in the ethanol-specific group: SGF-3 and NRF-1, which are an anti-oxidant and a stress response factor respectively (Fig. 5C). Our findings showing that the ethanol-specific group has more transcriptional co-regulators suggest that SGF-3 and NRF-1 may be acting to impair rather than enhance C/EBP regulation in the context of liver regeneration.
Known and Novel Motifs in Dynamic Patterns Revealed through Comparative Motif Analysis
Our results indicate that SMAD3 is a highly occupied motif during liver regeneration and ethanol adaptation. The ubiquity of SMAD3, the way it represses C/EBP, and the implications on TGF-β pathway regulation have been studied previously (7). In the context of liver regeneration and ethanol adaptation, SMAD3 may act to inhibit the effects of C/EBP binding.
We found C/EBP-β binding to loci with ATF binding sites in the samples from ethanol-treated animals post-PHx. Both ATF and C/EBP families belong to a class of bZIP proteins, and cross talk between these families through CREB/ATF family heterodimers can activate genes important to development (59). Our analyses showed ATF/CREB motifs in C/EBP-β binding in response to PHx, as well as C/EBP-α unbinding in response to PHx.
Our results show that the HNF4 motif was carbohydrate specific during C/EBP-α unbinding (Fig. 5A). The specific motif we found, however, is also a known motif of C/EBP and hence is difficult to attribute specifically to HNF4 or C/EBP binding without additional experimental data. Of relevance, HNF, C/EBP, and DBP have been shown to bind co-operatively in humans and rats, and the interaction of HNFs with C/EBPs have been reported in other species as well (55).
DISCUSSION
In this study, we investigated the binding patterns of C/EBP transcription factors during the priming phase of liver regeneration in ethanol-fed rats and isocaloric controls. We found that chronic ethanol intake led to alteration of the C/EBP response to PHx in several ways. Adaptation to chronic ethanol intake resulted in altered binding site selection by C/EBP-α and C/EBP-β, altered binding ratios of the C/EBPs before and following resection, mistimed binding of C/EBP-α and C/EBP-β following resection, and ethanol-specific or ethanol-missing C/EBP regulation of target genes. These ethanol-induced alterations to C/EBP binding characteristics in the priming phase of regeneration likely contribute to ethanol-mediated suppression of liver regeneration following PHx.
Our genome-wide analysis of C/EBP binding post PHx showed that, in control animals, C/EBP-β peak binding loci occurred upstream of the TSS, while C/EBP-α peak binding loci occurred downstream of the TSS. Following the combined insults of chronic ethanol adaptation and PHx, however, the localization of peak binding relative to TSS was reversed. Chronic ethanol adaptation caused peak C/EBP-β binding downstream of the TSS and peak C/EBP-α binding upstream of the TSS. This shift in binding distributions may indicate a change in the nature of C/EBP binding to target genes following chronic ethanol abuse. For example, C/EBP binding in control animals may prime target genes for transcription later during the replication phase while C/EBP binding in ethanol-fed animals may be regulating target genes actively in response to the ongoing stress of chronic ethanol use (52). Alternatively, the distinct binding locus distributions could result in a shift between activation and inhibition of target genes, thus promoting a different functional program of gene expression. Further studies are necessary to determine if either of these possibilities occurs.
Previous studies have explored the binding activity and expression of C/EBP-β with ethanol intake (5, 6, 22, 53). These studies suggest that ethanol intake affects C/EBP-β binding and inhibits C/EBP-β expression. Previous studies have also showed that, in healthy livers, C/EBP-α is expressed at higher levels than C/EBP-β (18). We found that the chronic ethanol adaptation caused an increase in overall binding of both C/EBP-α and C/EBP-β before PHx. C/EBP-α binding increased more than C/EBP-β binding, leading to an overall increase in the C/EBP-α to C/EBP-β binding ratio. This increased C/EBP-α/β ratio has been implicated previously in the termination of liver regeneration, where artificially decreasing C/EBP-α activation resulted in enhanced mass recovery (30). We hypothesize that the ethanol-mediated increase in C/EBP-α-to-C/EBP-β genome activity ratio may act as an early termination signal suppressing liver regeneration, consistent with the expected effects of such a shift in the C/EBP-α-to-C/EBP-β genome binding activity ratio (18).
In addition to altering the amount and the loci of C/EBP binding, chronic ethanol adaptation also altered the dynamics of C/EBP binding to target genes post-PHx. Our studies revealed that at 6 h post-PHx there was an overall increase in C/EBP-α binding compared with baseline in both ethanol-fed rats and controls. C/EBP-β binding at 6 h post-PHx, however, showed a larger binding response to PHx than C/EBP-α in ethanol-fed rats. Furthermore, the C/EBP-β binding response to PHx at 6 h was also stronger in ethanol-fed rats than controls. It is possible that this ethanol-mediated increase in C/EBP-β binding response is a compensatory response to the damage induced to the liver by a combination of chronic ethanol and PHx. An alternative explanation is that these changes in C/EBP-β DNA binding activity associated with ethanol adaptation contribute to the suppression of the hepatocyte cell cycle, or they could be a symptom of hepatocytes failing to progress beyond the G1 phase of the cell cycle.
Our study revealed several interesting pathway-level associations of C/EBP binding. Hepatic regeneration is known to be regulated by EGF and TGF-β through C/EBP-β and C/EBP-α activities (48). Growth activation may involve participation of immediate early growth response genes as partners in C/EBP heterodimers. Our C/EBP-α-specific binding results showed that two oppositely acting growth factors, EGF and TGF-β, are C/EBP-α targets, indicating a complex relationship between C/EBP-α binding and growth factor regulation following PHx. Our results further revealed that chronic ethanol intake can potentially modulate adipogenesis through C/EBP-β-specific binding. We found several targets associated with fat cell differentiation (including Bbs4, Ctbp1, Rgs2, Itga6, Gsk3b, Nudt7, Ucp1) bound by C/EBP-β in ethanol-fed rats. This is consistent with earlier findings showing that ethanol shifts the redox state in the liver to modify fatty acid oxidation, leading to induction of adipogenic enzymes (9). In addition, we were interested in cell type-specific activation of C/EBPs. Previous work has shown that C/EBP-β acts as an antiapoptotic factor during stellate cell activation (3). Additionally, reduced expression of C/EBP-α and increased nuclear levels of C/EBP-β has been observed during stellate cell activation in rats (26). Furthermore, it has been shown that the C/EBP-β signaling pathway potentially mediates collagen production in stellate cells exposed to methanol (64). In our study, several genes related to collagen production in hepatic stellate cells were regulated specifically in ethanol-adapted rats (Adamts1, Col3a1, Edn1, Lrat, Sparc, Mmp12). These genes were bound and may be regulated by C/EBP-β after ethanol adaptation, thereby contributing to the inhibitory effects of ethanol adaptation to liver regeneration post-PHx. We were also interested to find several genes identified as important to liver regeneration and growth regulated by C/EBP-β alone in response to PHx in only the control rats (Nfkb2, Rbpj, Vcam1, Vegfc, E2f1, Bcl10). C/EBP-β occupancy on the promoters of pro-proliferative E2F-regulated growth-related genes is known to increase as a function of cell cycle progression (62). While our results indicate an increase in C/EBP-β binding after chronic ethanol intake, some of the important liver-specific genes that are regulated by C/EBP-β, including E2f1, show a control-specific C/EBP-β binding, indicating that ethanol may negatively affect regulation of key genes. Overall, we conclude that ethanol-adaptation modulates different sets of pathways for C/EBP-β and C/EBP-α post-PHx. Ethanol induced C/EBP-α regulation of genes associated with homeostasis, cell growth, adhesion, and membrane-related functions; while ethanol induced C/EBP-β regulation of targets associated with response to wounding, adipogenesis, protein kinase cascade, chromosome organization and apoptosis. Additionally, ethanol adaptation may induce C/EBP regulation of the TGF-β pathway, leading to an impaired regenerative response in hepatocytes. Our results showed ethanol-specific baseline C/EBP-α and 6 h post-PHx C/EBP-β binding to genes associated with activation of the TGF-β pathway. This finding is consistent with recent work showing involvement of C/EBP in the activation of antiproliferative TGF-β signaling (41). We hypothesize that this regulation contributes to attenuated regeneration through enhanced TGF-β production and signaling in hepatocytes.
Finally, we used a comparative dynamic pattern counts (COMPACT) analysis as a novel way to explore further the ethanol-induced changes to C/EBP binding dynamics post-PHx. This layered approach revealed dominant patterns of dynamically altered binding to transcription factor-specific targets and diet-specific targets. By masking the dominant patterns, we uncovered underlying subtle patterns that are composed of smaller clusters of ethanol-altered C/EBP binding to target genes. These analyses revealed pathways where ethanol alters C/EBP regulation post-PHx, including enhanced C/EBP-α regulation of the TGF-β pathway, enhanced C/EBP-β regulation of response to wounding, and impaired C/EBP-β regulation of apoptosis and fatty acid metabolic processes. Our novel comparative method is flexible enough to be applicable to time series studies involving transcription factor binding, microRNA binding, and gene expression.
GRANTS
Financial support for this study was provided by National Institute on Alcohol Abuse and Alcoholism Grants R21 AA-016919, R01 AA-018873, R21 AA-022417, T32 AA-007463, F31 AA-023445.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.K., B.P., D.C., and R.V. analyzed data; L.K., B.P., D.C., J.B.H., and R.V. interpreted results of experiments; L.K. and R.V. prepared figures; L.K. and R.V. drafted manuscript; L.K., B.P., D.C., J.B.H., and R.V. edited and revised manuscript; L.K., B.P., D.C., J.B.H., and R.V. approved final version of manuscript; B.P. performed experiments.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Aalap Verma for assistance uploading the data generated during this study to the NCBI Signal Read Archive.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Anderson ER, Taylor M, Xue X, Martin A, Moons DS, Omary MB, Shah YM. The hypoxia-inducible factor-C/EBPα axis controls ethanol-mediated hepcidin repression. Mol Cell Biol 32: 4068–4077, 2012. doi: 10.1128/MCB.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 96: 447–455, 1995. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc 120: 361–368, 2009. [PMC free article] [PubMed] [Google Scholar]
- 4.Buck M, Chojkier M. Signal transduction in the liver: C/EBPbeta modulates cell proliferation and survival. Hepatology 37: 731–738, 2003. doi: 10.1053/jhep.2003.50155. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Kunos G, Gao B. Ethanol rapidly inhibits IL-6-activated STAT3 and C/EBP mRNA expression in freshly isolated rat hepatocytes. FEBS Lett 457: 162–168, 1999. doi: 10.1016/S0014-5793(99)01031-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Yang CM, Chang SP, Hu ML. C/EBP beta and C/EBP delta expression is elevated in the early phase of ethanol-induced hepatosteatosis in mice. Acta Pharmacol Sin 30: 1138–1143, 2009. doi: 10.1038/aps.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 278: 9609–9619, 2003. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- 8.Cook D, Ogunnaike BA, Vadigepalli R. Systems analysis of non-parenchymal cell modulation of liver repair across multiple regeneration modes. BMC Syst Biol 9: 71, 2015. doi: 10.1186/s12918-015-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correnti JM, Juskeviciute E, Swarup A, Hoek JB. Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am J Physiol Gastrointest Liver Physiol 306: G959–G973, 2014. doi: 10.1152/ajpgi.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crumm S, Cofan M, Juskeviciute E, Hoek JB. Adenine nucleotide changes in the remnant liver: An early signal for regeneration after partial hepatectomy. Hepatology 48: 898–908, 2008. doi: 10.1002/hep.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diehl AM, Chacon M, Wagner P. The effect of chronic ethanol feeding on ornithine decarboxylase activity and liver regeneration. Hepatology 8: 237–242, 1988. doi: 10.1002/hep.1840080208. [DOI] [PubMed] [Google Scholar]
- 12.Diehl AM, Yang SQ. Regenerative changes in C/EBP α and C/EBP β expression modulate binding to the C/EBP site in the c-fos promoter. Hepatology 19: 447–456, 1994. doi: 10.1002/hep.1840190225. [DOI] [PubMed] [Google Scholar]
- 13.Diehl AM. Roles of CCAAT/enhancer-binding proteins in regulation of liver regenerative growth. J Biol Chem 273: 30843–30846, 1998. doi: 10.1074/jbc.273.47.30843. [DOI] [PubMed] [Google Scholar]
- 14.Duguay L, Coutu D, Hetu C, Joly JG. Inhibition of liver regeneration by chronic alcohol administration. Gut 23: 8–13, 1982. doi: 10.1136/gut.23.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43, Suppl 1: S45–S53, 2006. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 16.Flodby P, Antonson P, Barlow C, Blanck A, Porsch-Hällström I, Xanthopoulos KG. Differential patterns of expression of three C/EBP isoforms, HNF-1, and HNF-4 after partial hepatectomy in rats. Exp Cell Res 208: 248–256, 1993. doi: 10.1006/excr.1993.1244. [DOI] [PubMed] [Google Scholar]
- 17.Friedman JR, Larris B, Le PP, Peiris TH, Arsenlis A, Schug J, Tobias JW, Kaestner KH, Greenbaum LE. Orthogonal analysis of C/EBPbeta targets in vivo during liver proliferation. Proc Natl Acad Sci USA 101: 12986–12991, 2004. doi: 10.1073/pnas.0402875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenbaum LE, Cressman DE, Haber BA, Taub R. Coexistence of C/EBP alpha, beta, growth-induced proteins and DNA synthesis in hepatocytes during liver regeneration. Implications for maintenance of the differentiated state during liver growth. J Clin Invest 96: 1351–1365, 1995. doi: 10.1172/JCI118170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R. CCAAT enhancer- binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest 102: 996–1007, 1998. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grøntved L, John S, Baek S, Liu Y, Buckley JR, Vinson C, Aguilera G, Hager GL. C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J 32: 1568–1583, 2013. doi: 10.1038/emboj.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem 281: 22974–22982, 2006. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 23.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis 24: 257–272, 2004. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang G-C, Zhang J-S, Tang Q-Q. Involvement of C/EBP-alpha gene in in vitro activation of rat hepatic stellate cells. Biochem Biophys Res Commun 324: 1309–1318, 2004. doi: 10.1016/j.bbrc.2004.09.196. [DOI] [PubMed] [Google Scholar]
- 27.Ishiyama T, Kano J, Minami Y, Iijima T, Morishita Y, Noguchi M. Expression of HNFs and C/EBP alpha is correlated with immunocytochemical differentiation of cell lines derived from human hepatocellular carcinomas, hepatoblastomas and immortalized hepatocytes. Cancer Sci 94: 757–763, 2003. doi: 10.1111/j.1349-7006.2003.tb01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobsen JS, Waage J, Rapin N, Bisgaard HC, Larsen FS, Porse BT. Temporal mapping of CEBPA and CEBPB binding during liver regeneration reveals dynamic occupancy and specific regulatory codes for homeostatic and cell cycle gene batteries. Genome Res 23: 592–603, 2013. doi: 10.1101/gr.146399.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji H, Jiang H, Ma W, Johnson DS, Myers RM, Wong WH. An integrated software system for analyzing ChIP-chip and ChIP-seq data. Nat Biotechnol 26: 1293–1300, 2008. doi: 10.1038/nbt.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin J, Hong I-H, Lewis K, Iakova P, Breaux M, Jiang Y, Sullivan E, Jawanmardi N, Timchenko L, Timchenko NA. Cooperation of C/EBP family proteins and chromatin remodeling proteins is essential for termination of liver regeneration. Hepatology 61: 315–325, 2015. doi: 10.1002/hep.27295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson PF. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118: 2545–2555, 2005. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- 32.Juskeviciute E, Vadigepalli R, Hoek JB. Temporal and functional profile of the transcriptional regulatory network in the early regenerative response to partial hepatectomy in the rat. BMC Genomics 9: 527–552, 2008. doi: 10.1186/1471-2164-9-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang L-I, Mars WM, Michalopoulos GK. Signals and cells involved in regulating liver regeneration. Cells 1: 1261–1292, 2012. doi: 10.3390/cells1041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koteish A, Yang S, Lin H, Huang J, Diehl AM. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res 26: 1710–1718, 2002. doi: 10.1111/j.1530-0277.2002.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 35.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res 19: 1639–1645, 2009. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuttippurathu L, Juskeviciute E, Dippold RP, Hoek JB, Vadigepalli R. A novel comparative pattern analysis approach identifies chronic alcohol mediated dysregulation of transcriptomic dynamics during liver regeneration. BMC Genomics 17: 260, 2016. doi: 10.1186/s12864-016-2492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuttippurathu L, Patra B, Hoek JB, Vadigepalli R. A novel comparative pattern count analysis reveals a chronic ethanol-induced dynamic shift in immediate early NF-κB genome-wide promoter binding during liver regeneration. Mol Biosyst 12: 1037–1056, 2016. doi: 10.1039/C5MB00740B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol 233: 585–594, 1994. doi: 10.1016/S0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- 39.Mahony S, Benos PV. STAMP: a web tool for exploring DNA-binding motif similarities. Nucleic Acids Res 35, Suppl 2: W253–W258, 2007. doi: 10.1093/nar/gkm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Münch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31: 374–378, 2003. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalopolous G. Terminating hepatocyte proliferation during liver regeneration: the roles of two members of the same family (CCAAT-enhancer-binding protein alpha and beta) with opposing actions. Hepatology 61: 32–34, 2015. doi: 10.1002/hep.27329. [DOI] [PubMed] [Google Scholar]
- 43.Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol 17: 318–324, 2007. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Payne VA, Au W-S, Lowe CE, Rahman SM, Friedman JE, O’Rahilly S, Rochford JJ. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem J 425: 215–223, 2009. doi: 10.1042/BJ20091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polager S, Ginsberg D. E2F - at the crossroads of life and death. Trends Cell Biol 18: 528–535, 2008. doi: 10.1016/j.tcb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365: 561–575, 2002. doi: 10.1042/bj20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rana B, Xie Y, Mischoulon D, Bucher NL, Farmer SR. The DNA binding activity of C/EBP transcription factor is regulated in the G1 phase of the hepatocyte cell cycle. J Biol Chem 270: 18123–18132, 1995. doi: 10.1074/jbc.270.30.18123. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt D, Wilson MD, Ballester B, Schwalie PC, Brown GD, Marshall A, Kutter C, Watt S, Martinez-Jimenez CP, Mackay S, Talianidis I, Flicek P, Odom DT. Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science 328: 1036–1040, 2010. doi: 10.1126/science.1186176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol Rev 54: 129–158, 2002. doi: 10.1124/pr.54.1.129. [DOI] [PubMed] [Google Scholar]
- 51.Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol Rev 56: 291–330, 2004. doi: 10.1124/pr.56.2.5. [DOI] [PubMed] [Google Scholar]
- 52.Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, Young RA, Sharp PA. Divergent transcription from active promoters. Science 322: 1849–1851, 2008. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seth D, Gorrell MD, Cordoba S, McCaughan GW, Haber PS. Intrahepatic gene expression in human alcoholic hepatitis. J Hepatol 45: 306–320, 2006. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Smith AD, Sumazin P, Das D, Zhang MQ. Mining ChIP-chip data for transcription factor and cofactor binding sites. Bioinformatics 21, Suppl 1: i403–i412, 2005. doi: 10.1093/bioinformatics/bti1043. [DOI] [PubMed] [Google Scholar]
- 55.Szpirer C, Riviere M, Cortese R, Nakamura T, Islam MQ, Levan G, Szpirer J. Chromosomal localization in man and rat of the genes encoding the liver-enriched transcription factors C/EBP, DBP, and HNF1/LFB-1 (CEBP, DBP, and transcription factor 1, TCF1, respectively) and of the hepatocyte growth factor/scatter factor gene (HGF). Genomics 13: 293–300, 1992. doi: 10.1016/0888-7543(92)90245-N. [DOI] [PubMed] [Google Scholar]
- 56.Takayama K, Kawabata K, Nagamoto Y, Inamura M, Ohashi K, Okuno H, Yamaguchi T, Tashiro K, Sakurai F, Hayakawa T, Okano T, Furue MK, Mizuguchi H. CCAAT/enhancer binding protein-mediated regulation of TGFβ receptor 2 expression determines the hepatoblast fate decision. Development 141: 91–100, 2014. doi: 10.1242/dev.103168. [DOI] [PubMed] [Google Scholar]
- 57.Timchenko NA, Wilde M, Darlington GJ. C/EBPalpha regulates formation of S-phase-specific E2F-p107 complexes in livers of newborn mice. Mol Cell Biol 19: 2936–2945, 1999. doi: 10.1128/MCB.19.4.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vadigepalli R, Chakravarthula P, Zak DE, Schwaber JS, Gonye GE. PAINT: a promoter analysis and interaction network generation tool for gene regulatory network identification. OMICS 7: 235–252, 2003. doi: 10.1089/153623103322452378. [DOI] [PubMed] [Google Scholar]
- 59.Vallejo M, Ron D, Miller CP, Habener JF. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci USA 90: 4679–4683, 1993. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wands JR, Carter EA, Bucher NL, Isselbacher KJ. Inhibition of hepatic regeneration in rats by acute and chronic ethanol intoxication. Gastroenterology 77: 528–531, 1979. [PubMed] [Google Scholar]
- 61.Wang B, Gao C, Ponder KP. C/EBPbeta contributes to hepatocyte growth factor-induced replication of rodent hepatocytes. J Hepatol 43: 294–302, 2005. doi: 10.1016/j.jhep.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Larris B, Peiris TH, Zhang L, Le Lay J, Gao Y, Greenbaum LE. C/EBPbeta activates E2F-regulated genes in vivo via recruitment of the coactivator CREB-binding protein/P300. J Biol Chem 282: 24679–24688, 2007. doi: 10.1074/jbc.M705066200. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Peiris TH, Mowery A, Le Lay J, Gao Y, Greenbaum LE. CCAAT/enhancer binding protein-beta is a transcriptional regulator of peroxisome-proliferator-activated receptor-gamma coactivator-1alpha in the regenerating liver. Mol Endocrinol 22: 1596–1605, 2008. doi: 10.1210/me.2007-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J-H, Batey R-G, George J. Role of ethanol in the regulation of hepatic stellate cell function. World J Gastroenterol 12: 6926–6932, 2006. doi: 10.3748/wjg.v12.i43.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Attard FA, Tankersley LR, Potter JJ, Mezey E. Effect of retinoic acid on the enhancing effect of acetaldehyde on mouse type I collagen expression. Arch Biochem Biophys 376: 191–198, 2000. doi: 10.1006/abbi.2000.1723. [DOI] [PubMed] [Google Scholar]
- 66.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 277: 29342–29347, 2002. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 67.Zhang BH, Hornsfield BP, Farrell GC. Chronic ethanol administration to rats decreases receptor-operated mobilization of intracellular ionic calcium in cultured hepatocytes and inhibits 1,4,5-inositol trisphosphate production: relevance to impaired liver regeneration. J Clin Invest 98: 1237–1244, 1996. doi: 10.1172/JCI118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137, 2008. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu LJ, Gazin C, Lawson ND, Pagès H, Lin SM, Lapointe DS, Green MR. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics 11: 237–247, 2010. doi: 10.1186/1471-2105-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.