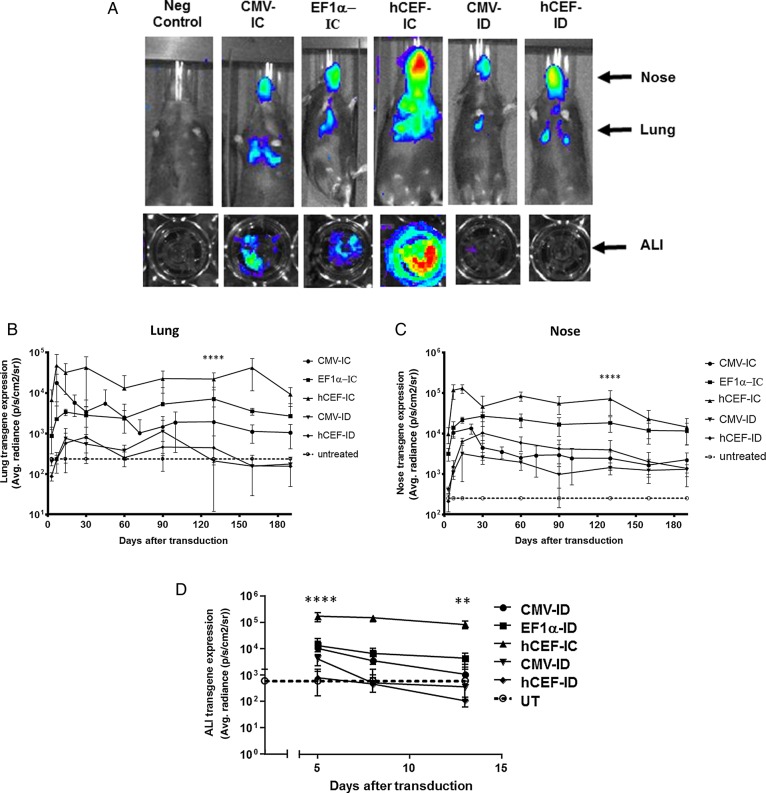
Figure 1.
Selection of lead candidate vector. Mice and human air–liquid interface (ALI) cultures were transduced with five different lentiviral vector configurations by nasal instillation: integrase-competent (IC) vectors carrying the human elongation factor 1α (short) promoter (EF1α),23 a ubiquitous regulatory element which has previously been used in the context of lentivirus-mediated gene transfer,48 an in-house, synthetic chimeric promoter/enhancer consisting of CpG-depleted versions of the human EF1α (short) promoter and the human cytomegalovirus (CMV) enhancer (hCEF)24 and the original CMV-based construct, as well as integrase-defective (ID) vectors carrying the CMV promoter and hCEF promoter/enhancers (6–30E7 TU/mouse or ALI, n=6–10 mice/group, n=4 ALI/group). All vectors carried a luciferase reporter gene for quantification of gene expression by bioluminescence imaging. Negative control mice and ALIs remained untreated (UT). Gene expression was quantified in the lungs and nose of mice and in ALIs. Photon emission adjusted for differences in vector titre. (A) Representative images of transduced and UT mice and ALIs, (B) quantification of photon emission in murine lungs, (C) quantification of photoemission in murine nose and (D) in human ALIs. (B–D) Reference UT control values are shown as a dotted line (lung control: 182±6 p/s/cm2/sr, nose control: 200±10 p/s/cm2/sr, ALI control 598±1080 p/s/cm2/sr). For each group, the mean±SEM are shown. ****p<0.001 in lung and nose comparing hCEF-ID with all other vectors in mice (ANOVA followed by Tukey post hoc test), ***p<0.005 comparing hCEF-IC with UT ALI controls (Mann–Whitney).