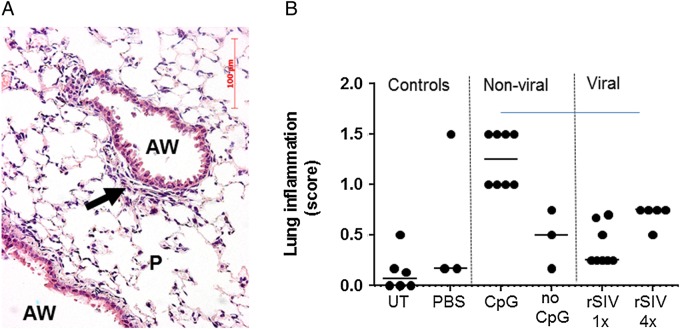
Figure 4.
Assessment of acute pulmonary toxicity after lentivirus transduction. Mice were transduced with one or four doses (1E8 TU/dose at monthly intervals, n=5/group) of rSIV.F/HN-cytomegalovirus vectors carrying luciferase or enhanced green fluorescent protein reporter genes and histological analysis was performed 24 hours after the last dose. Control groups included UT and D-PBS-treated mice and mice treated with conventional (CpG containing) luciferase plasmid DNA/GL67A complexes or CpG-free CFTR plasmid pGM169/GL67A (n=3–5 mice/group). (A) Representative image of a lentivirus-treated mouse. AW, airway, P, parenchyma, arrow indicates mild cellular infiltrate, (B) semiquantitative scoring of lung inflammation. UT, untreated, one dose of rSIV (rSIV1x) and four monthly doses of rSIV (rSIV4x). Each symbol represents an individual mouse. The horizontal bar indicates the group median.